Abstract
Per- and polyfluoroalkyl substances (PFASs) are key ingredients of firefighting foams designed to suppress fires involving flammable and combustible liquids. Such foams are used by firefighters during fire training at dedicated sites. Because PFASs are very persistent chemicals, substantial soil and groundwater contamination has been observed in the vicinity of firefighter training areas. However, very few data are available on PFAS contamination of wastewater and runoff water on such sites. The purpose of this study was to evaluate the occurrence of more than 50 PFASs in 43 water samples (effluent from a wastewater treatment plant (WWTP), lagoon, runoff water, and wastewater drained from firefighter training areas) collected from a large firefighter training site, using target and suspect screening. A comparison of the PFAS classes analyzed revealed the overwhelming contribution of fluorotelomers. This indicates that the PFAS emission from the use of firefighting foams cannot be monitored only by measuring perfluoroalkyl acids. Based on the PFAS concentrations measured through target screening, the PFAS mass discharged into the river receiving WWTP effluent and the lagoon was on average 387 ± 183 kg and 56 ± 15 kg per year respectively. Due to the unavailability of standards, it was impossible to take into account the PFASs detected with suspect screening. The present study emphasizes that, above and beyond soil and groundwater contamination, such sites also contribute to the PFAS burden of surface water.
Similar content being viewed by others
Class B firefighting foams are designed to suppress fires involving flammable and combustible liquids, such as liquid hydrocarbon fuels and polar solvents where the fire remains on the surface of the liquid. These foams are formulated to float on flammable liquids, and prevent reignition by excluding air and cooling the hot surface (Kissa 2001). It appears that per- and polyfluoroalkyl substances (PFASs) have been included in such foams since 1966 (Place and Field 2012; Prevedouros et al. 2006). Perfluoroalkyl substances, such as perfluoroalkane sulfonic acids (PFSAs, CnF2n+1SO3H) or perfluoroalkyl carboxylic acids (PFCAs, CnF2n+1CO2H), are compounds in which all the carbon atoms in the main chain are fluorinated (Buck et al. 2011). The C–F bond being one of the strongest thermodynamic bonds known (Renner 2001), these substances are highly persistent under environmental conditions and ubiquitous in the global environment. Polyfluoroalkyl substances, such as perfluorooctane sulfonamidoacetic acid (FOSAA, C8F17SO2NHCH2COOH) or n:2 fluorotelomer sulfonic acids (n:2 FTSAs, CnF2n+1C2H4SO3H), are compounds in which some of the carbon atoms in the chain are not fully fluorinated (Buck et al. 2011). Consequently, they have the potential to transform under environmental conditions into perfluoroalkyl substances, their endpoint metabolites (Buck et al. 2011). Fluorotelomers (FTs) are a subgroup of polyfluoroalkyl substances with an unfluorinated “spacer” (usually –C2H4–) between the perfluorinated tail (CnF2n+1) and a functional group (Buck et al. 2011). 6:2 Fluorotelomer sulfonamide alkylbetaine (6:2 FTAB, C6F13C2H4S(O)2N(H)C3H6N(CH3)2CH2CO2H) is an example of an FT.
Before the progressive market withdrawal of PFOS-based products (starting from May 2000) and their subsequent regulation by various authorities, the predominant surfactants produced and sold worldwide for firefighting foam formulations were PFOS-based (Prevedouros et al. 2006). Approximately 10% of all PFOS-based chemical production between 1970 and 2002 (i.e., 96,000 tons) went into firefighting foams (Paul et al. 2009). FT-based foams were available in the 1970s and gradually extended their market share with the phase-out of PFOS-based products (Prevedouros et al. 2006). In Europe, PFOS-based foams were regulated by Directive 2006/122/EC (2006). By way of derogation, if these foams had been placed on the market before December 27, 2006, they could be used until June 27, 2011.
Class B firefighting foams are used to extinguish major accidental fires (Oakes et al. 2010; Taniyasu et al. 2015; Weber et al. 2010) but also routinely during fire training exercises at dedicated training centers, airports, or oil refineries (Baduel et al. 2017; Filipovic et al. 2015; Kärrman et al. 2011; Weber et al. 2017). These releases lead to contamination by PFASs of biota (de Vries et al. 2017; Gewurtz et al. 2014), groundwater (Backe et al. 2013; Schultz et al. 2004), and soil (Houtz et al. 2013; McGuire et al. 2014) in the vicinity of such sites. However, no studies have estimated the amount of PFASs released into the environment through runoff water and the sewers of operational training sites.
The present study focused on a major firefighter training center in France. Because the groundwater and soil contamination by PFASs had already been investigated (Dauchy et al. 2019), the purpose of this study was to evaluate PFAS load drained from the firefighting ground and surface soil and found in wastewater and runoff water. To address this issue, samples (n = 43) were collected from different water systems on the site (sewers, lagoon, and a wastewater treatment plant) and 31 PFASs were analyzed by high-performance liquid chromatography coupled with tandem mass spectrometry. Additionally, suspect screening was performed on selected samples to detect PFASs known to be key ingredients of Class B firefighting foams. No analytical standards were used, however, as none were available.
Materials and Methods
Site Description
The site covers a fenced area of 2.4 km2 where firefighting foams have been used intensively. From 1969 to 1984, this site was an oil refinery. The exact location of the training area, the frequency of training sessions, and the history of firefighter training activities are unknown within that period. From 1987 to date, it has become a large training area for firefighters. The exercises were originally performed directly on the soil, but from the 1990s onward, some of the exercise areas were covered with concrete. The annual quantities of foam used between 1987 and 2006 were not recorded, but between 2006 and 2015, 20–40 m3 of foam concentrates per year were used for the exercises (29 m3 on average). PFASs are present in foam concentrates at a level of 1–5% by weight (Moody and Field 2000; Moody et al. 2002; Scheffey and Hanauska 2002; SFT 2008), and the approximate density of the concentrate is equal to 1 kg/L according to some material safety data sheets (MSDSs). On the basis of this information, the total quantity of PFASs released into the environment from this site ranged from 290 to 1450 kg per year. It is impossible to be more precise regarding the fluorinated ingredients contained in the foams used on this site, since the available MSDSs did not provide this information. However, it is quite likely that foams containing PFSA precursors as their main ingredient were used onsite until at least 2011.
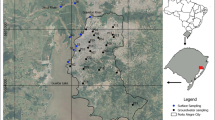
The site has three concrete slabs currently used for fire training (Fig. 1). The wastewater from firefighting exercises drains from these slabs into a sewer system (Supplemental Fig. S1) and is then treated in a wastewater treatment plant (WWTP) based on a conventional coagulation-flocculation-sedimentation treatment train. The effluent is then discharged into a river. Runoff water from the rest of the site (approximately 1.9 km2) is collected via a dedicated system (Fig. 1) and discharged into a lagoon. During firefighting exercises, the water used to make the aqueous foam solution from concentrates is pumped from the lagoon. Excess water from the lagoon is discharged into the same river as the WWTP effluent.
Scheme of the runoff water network (RW) and location of the sampling points. For clarity, the network was drawn with three colors: the purple network drains runoff water mainly from the north-eastern part of the site; the red one drains runoff water mainly from the southeastern part of the site, and the light blue one drains runoff water from the western part of the site. The three shaded areas represent the location of the places currently used for firefighter training sessions. The sampling point labels are colored according to the color scale below, related to total concentrations of quantified PFASs (ng/L)
Sampling
A total of 43 water samples were collected in July 2015 and October 2016: 4 of wastewater from firefighting exercises (TW), 7 of effluent from the WWTP (WW), 22 of runoff water (RW), 6 of lagoon water (L), and 4 of water used for diluting foam concentrates (WFF). Figures 1 and S1 depict the location of all the sampling points.
All of the samples were collected in new wide-mouth plastic bottles [1-L high-density polyethylene (HDPE)]. They were returned to the laboratory in chilled cool boxes to minimize losses due to degradation and stored at 4 °C before analysis. Samples were analyzed within 2 weeks of collection.
Chemicals
The target PFASs included 11 PFCAs (CnF2n+1COO−, n = 3–13), 8 PFSAs (CnF2n+1SO3−, n = 4, 6, 7, 8, 10), 3 n:2 FTSAs (CnF2n+1C2H4SO3−, n = 4, 6, 8), 3 fluorotelomer carboxylic acids (n:2 FTCAs, CnF2n+1 CH2COO−, n = 6, 8, 10), 3 fluorotelomer unsaturated carboxylic acids (n:2 FTUCAs, CnF2nCHCOO−, n = 6, 8, 10), 5:3 ACID (C5F11(CH2)2COO−), perfluorooctane sulfonamide (FOSA, C8F17SO2NH2), FOSAA, N-Methyl perfluorooctane sulfonamidoacetic acid (MeFOSAA, C8F17SO2N(CH3)CH2COOH), N-Ethyl perfluorooctane sulfonamidoacetic acid (EtFOSAA, C8F17SO2N(C2H5)CH2COOH), 6:2 FTAB, and 6:2 Fluorotelomer sulfonamide propyl N,N-dimethylamine (6:2 FtSaAM, C6F13C2H4S(O)2N(H)C3H6N(CH3)2). These compounds were monitored in all the collected samples and quantified using 13 mass-labeled standards. Their chemical source and purity are given in Boiteux et al. (2016). All solvents and reagents were high-purity grade. 6:2 FTAB and 6:2 FtSaAM standards were gifted by a manufacturer.
Analysis
Water samples were processed and analyzed using protocols previously described (Boiteux et al. 2016). Briefly, nonfiltered water samples (250 mL) were spiked with labeled internal standards and concentrated by solid phase extraction (SPE) (cartridge Strata X-AW 200 mg/6 mL; Phenomenex®, France). Offline enrichment was conducted on an automated system (GX-274, Aspec; Gilson, France). PFASs were eluted from the cartridges by the successive use of 1 mL of methanol (MeOH), 4 mL of ammonium hydroxide (0.1% (v/v) (NH4OH) in MeOH, and 2 mL of NH4OH 0.1% (v/v) in isopropanol/dichloromethane (30/70) before being combined and concentrated under a nitrogen stream to a final volume of 100 µL.
Chromatographic separation was performed by a Waters Acquity UPLC® liquid chromatograph (Waters, Guyancourt, France), and analytes were detected by a mass spectrometer interfaced with an electrospray ionization source (Waters Xevo TQ-MS). To achieve better limits of quantification (LOQs) for 6:2 FTAB and 6:2 FtSaAM, chromatographic separation and detection were performed by a 20AD liquid chromatograph (Shimadzu, Marne-la-Vallée, France) coupled with a quadrupole ion trap mass spectrometer 5500 QTRAP instrument (AB SCIEX, Villebon-sur-Yvette, France). Compound-dependent acquisition parameters were optimized by infusing PFAS standards. They were published, along with performance of the analytical method, in Boiteux et al. (2016) and are available in Supplemental Tables S1 and S2. Briefly, the recoveries from the water samples were typically in the 70–120% range and LOQs in the 4–25 ng/L range.
Initially, a list of 124 PFASs potentially present in Class B firefighting foams was monitored using ion transitions given in the literature. This comprehensive screening was performed on foam concentrates used on this site (Dauchy et al. 2017b) and the top layer of the most contaminated soil core (Dauchy et al. 2019). Based on the outcomes of these studies, 25 PFASs and transformation products were selected for the suspect screening performed in this study (Supplemental Table S3) and sought using the same offline enrichment as described for the targeted PFASs before injection on the Waters system. Neither authentic analytical standards nor reference materials were available, so these analytes were not quantified. Instead of estimating their concentrations by assuming a hypothetic equal molar response to a related standard, the presence of these PFASs was expressed as an integrated peak area of the analyte. Because it was impossible to estimate the recoveries of these PFASs with the analytical method used, only water samples with high targeted-PFAS concentrations were analyzed through suspect screening. Thus, two samples of firefighting exercise wastewater (TW-2 and TW-3), two of WWTP effluent (WW-2 and WW-5), and four of runoff water (RW-1, RW-3, RW-15 and RW-16) were analyzed.
Results and Discussion
Wastewater Drained from the Fire-Training Areas (TW)
Total concentrations of quantified PFASs ranged from 5.3 × 106 to 1.2 × 108 ng/L (Supplemental Table S4) and were consistent with the range of fluorocarbon surfactant concentrations (3.0 × 108–3.9 × 109 ng/L, detailed calculations are provided in the supporting information) given in some aqueous foam solutions (Moody and Field 2000; Scheffey and Hanauska 2002; SFT 2008). These concentrations in the TW-2 and TW-3 samples were 50-fold higher than those measured in TW-1 and TW-4. The TW-2 and TW-3 sampling points were located near two exercise areas. Thus, the high PFAS concentrations observed are not surprising and indicate that training sessions were performed recently, after which a large portion of the foams used during training sessions drains into the wastewater network. Because training sessions are regularly performed near the TW-1 sampling point, the lower PFAS concentration measured (compared to TW-2 and TW-3) indicates that foams are probably not well drained in this area. The TW-4 sampling point was located much further downstream in the wastewater network (near the WWTP). Therefore, the total PFAS concentration measured (5.3 × 106 ng/L) reflects the average PFAS load arriving at the WWTP and the dilution by other sewers connected to the network.
In these four samples, the fluorotelomer contribution to the total PFAS concentration was overwhelming (86.6–97.4%) and mainly accounted for by 6:2 FTAB. Only this fluorotelomer—a key ingredient of foams—and its main metabolites (6:2 FTSA and 6:2 FtSaAM) were quantified due to the unavailability of analytical standards for the many other fluorotelomers used as key ingredients in foams (Barzen-Hanson et al. 2017). The suspect screening of samples TW-2 and TW-3 revealed the presence of many PFASs (Table 1). Hence, two homologues of 6:2 FTAB (8:2 and 10:2 FTAB) and four by-products of this fluorotelomer class were identified (4:2 and 8:2 FtSaAM, 6:2 FtSa and 6:2 FtSaMAm). Several homologues of four other fluorotelomer classes (n:1:2 FtB, n:3 FtB, n:2 FTSAS, n:2 FtTNH+), and their related by-products were also detected. Major discrepancies between the peak areas measured for several fluorotelomers suggested that different foams were used on the two firefighter training areas. Thus, the peak areas of 6:2 FtTNH+, 6:2 FTSAS and 7:3 FtB in TW-3 were respectively 2.4, 2.7, and 11.8 higher than those in TW-2. Although it was impossible to quantify all the fluorotelomers detected, the overwhelming contribution of analyzed fluorotelomers to total PFAS concentration is obvious. This indicates that fluorotelomer-based foams are now predominantly used on this site and that the PFAS emission by this kind of activity cannot be monitored just by measuring perfluoroalkyl acids (PFAAs).
In these four samples, FOSA—a PFOS precursor—was systematically found, and the PFOS contribution to total PFSA concentration ranged from 60.3 to 81.8%. Comparisons with other studies were difficult due to the limited number of investigations under similar conditions. Hence, PFOS concentrations in TW-2 and TW-3 were similar to those reported in surface water samples (1.6 × 106–2.2 × 106 ng/L) collected a few days after the accidental release of foams at airports (Kwadijk et al. 2014; Moody et al. 2002). Yet even after a rapid and massive foam spill, the PFOS concentration in surface water is likely to be lower than in the wastewater due to dilution by the river. On the other hand, the PFOS and PFOA concentrations reported in the present study were lower than those found in an evaporation pond used to collect the wastewater from a firefighter training area where no PFOS-based foams were used for 5–6 years (3.6–9.7 × 106 ng/L for PFOS, 0.6–1.7 × 106 ng/L for PFOA) (Arias et al. 2014), but they were higher than those recorded in a drainage pond beside a firefighter training area on an airport where 15,000 L of PFOS-based foams were used each year between 1985 and 1994 (4.9 × 104–1.3 × 105 ng/L for PFOS, 4.7–7.0 × 103 ng/L for PFOA) (Bhavsar et al. 2016). From these comparisons, it could be assumed that PFOS-based foams were probably used a few years ago and that related PFASs are still leaching from the concrete surface of the firefighter training pads and the sewers draining the wastewater (Arias et al. 2014; Baduel et al. 2015). Suspect screening revealed the presence of four PFSA precursors (PFHxSA, PFHxSAm, PFOSAm, and PFHxSAmA) and two PFSAs (PFNS and PFPeS) (Table 1), confirming that PFSA-based foams had been used, although it was not possible to provide an approximate date of their last use.
Effluent from the Wastewater Treatment Plant (WW)
The total concentrations of quantified PFASs in the WWTP effluent ranged from 3.6 × 106 to 1.3 × 107 ng/L (Supplemental Table S5) and were thus consistent with the concentration measured in the sewer system just upstream of the WWTP inlet (5.3 × 106 ng/L at TW-4). This suggests that the wastewater treatment implemented on this site is not very efficient in removing PFASs. Furthermore, compared to TW-4, the relative contribution of 6:2 FTSA to total FT concentration did not significantly increase and that of 6:2 FTAB was even slightly higher (Supplemental Fig. S2), suggesting that there was no biotransformation of 6:2 FTAB or other FTs into 6:2 FTSA during the wastewater treatment. Supporting this assumption, only one metabolite of n:2 FTAB homologues (6:2 FtSaMAm) out of the four surveyed through suspect screening (Supplemental Table S6) was detected with relatively small peak areas (< 10,000). However, it is worth noting that peak area ratios between 6:2 FTSAS, 6:2 FtTHN+ and their related sulfoxides were lower in wastewater effluent than in wastewater drained from the firefighters’ exercise areas, indicating a relatively fast oxidation of both fluorotelomers in the sewer network and the WWTP.
Unlike 6:2 FTAB, the relative contribution of PFOS to the total PFSA concentration was lower in the effluent than in the sewer system (Supplemental Fig. S3). PFOS is known to be partially removed via sorption to sludge (Arvaniti et al. 2014). The average annual volume of effluent being about 50,000 m3, the total identified PFAS mass discharged into the receiving river was on average 387 ± 183 kg per year. This is 8–10-fold lower than the total PFAS mass discharged by some fluoropolymer manufacturing facilities (Bach et al. 2017), but only slightly lower than the total PFAS mass discharged by a fluorochemical manufacturing facility, estimated at between 600 and 2630 kg per year (Dauchy et al. 2017a). Firefighter training sites may thus be considered as PFAS sources releasing substantial levels into the environment. The suspect screening (Supplemental Table S6) revealed large discrepancies in peak areas between June (WW-2) and November 2015 (WW-5), suggesting that many more fire drills were performed in November 2015, and indicating that the PFAS mass load in the WWTP can change abruptly over time. No PFSA precursors except FHxSA were detected in the wastewater, suggesting that this type of foam was used on this site less frequently over time or was not used as intensively as on other investigated sites (Anderson et al. 2016; Arias et al. 2014; Baduel et al. 2017).
Runoff Water (RW)
Total concentrations of quantified PFASs in the runoff water network ranged from 2.9 × 107 to 1.0 × 103 ng/L (Fig. 2; Supplemental Table S7). The highest concentrations were recorded at sampling points RW-1 (2.9 × 107 ng/L), RW-16 (7.7 × 106 ng/L), and RW-15 (5.5 × 106 ng/L). These three points are all located near two areas where firefighter training sessions are performed. Because the concentrations were of the same order of magnitude as those observed in the sewer system, this suggests that the foams generated during exercises are not properly collected and drained from these areas (the training ground may be too narrow, or the slope of the concrete slab not properly designed, etc.). Consequently, a large amount of the foam resulting from exercises was found in the runoff water network and the soil (Dauchy et al. 2019). FOSA was quantified only in these three samples, because it was in wastewater drained from the firefighters’ exercise areas, further highlighting the similarity between these samples. Two PFSAs (FHxSA and PFPeS) were also observed in these three samples and RW-3 through suspect screening (Supplemental Table S8), confirming the systematic presence of this PFAS class and its precursors in the most contaminated areas of this site. However, the suspect screening also revealed that more PFASs and higher peak areas were observed in RW-1 and RW-15 than in RW-3 and RW-16 (Supplemental Table S8). This indicates that, despite some similarities at first glance, the analysis of many PFASs revealed some disparities in PFAS patterns that could not have been observed had only legacy PFASs been determined.
Near the training ground already highlighted by sampling point RW-1, the total concentration of quantified PFASs was 1.2 × 106 ng/L (RW-2), confirming the poor drainage of wastewater in this area. Downstream of these two training grounds (sampling points RW-3, RW-12, and RW-8), total concentrations of quantified PFASs were slightly lower due to dilution, ranging from 8.9 × 105 to 1.4 × 106 ng/l. Total concentrations of quantified PFASs ranging from 5.4 × 105 (RW-11) to 1.5 × 105 ng/L (RW-17) were recorded in areas where no firefighter exercises were supposed to have been performed (RW-6, RW-11, RW-17, RW-18, RW-19, RW-20, and RW-21) as well as in samples collected downstream of known firefighter training grounds (RW-4 and RW-7). The presence of PFASs at similar concentrations in both these kinds of areas suggests that either foams are/were spilled in areas not recorded or were insufficiently contained during exercises, leading to the contamination of adjacent surfaces. Total concentrations of quantified PFASs below 7.0 × 104 ng/L were observed in the upper parts of the runoff water network, in the areas furthest from the known exercise grounds (RW-9, RW-10, RW-13, RW-14, and RW-22).
The PFOS concentrations in a few samples (RW-7, RW-11, and RW-18) were similar to those observed in runoff water (around 7.0 × 103 ng/L) 1 month after the use of at least 40,000 L of foam to control a huge fuel-related fire in Japan (Taniyasu et al. 2015). However, the concentrations of the other PFASs measured could not be compared, suggesting that the PFAS profile observed on the firefighter training site stems from the use of many types of foams for many years, unlike the case of the petrochemical fire in Japan.
In runoff water samples, the relative contributions of PFSAs, PFCAs, precursors of PFOS, and FTs to the total PFAS concentration were similar to those observed in the wastewater drained from the firefighters’ exercise areas (Supplemental Fig. S4). However, in the remotest areas where the total PFASs were the lowest (RW-9, RW10, and RW-14), higher contributions of PFCAs and PFSAs were recorded. Due to their locations, it is likely that these sampling points are not frequently and intensively flushed and that PFASs remain longer in these points, promoting their transformation into PFCAs and PFSAs.
Lagoon (L) and Water Used for Diluting Foam Concentrates (WFF)
In the lagoon (Supplemental Table S5), total concentrations of quantified PFASs ranged from 2.3 × 105 to 5.0 × 105 ng/L, remained similar from one sampling time to another, and were consistent with the concentration measured in the runoff water network just upstream of the lagoon (RW-7). The relative contribution of FTs to the total PFAS concentration was always 94% (Supplemental Table S5), indicating that almost no transformation occurred in the lagoon.
The relative contribution of PFOS to the total PFSA concentration was systematically above 67%, similar to that measured in the runoff water network and higher than that observed in the WWTP (Supplemental Fig. S5), suggesting that this PFSA remains in the dissolved phase in the lagoon.
Every year, the maximum volume of water discharged into the river from the lagoon is set at 150,000 m3. With an average total PFAS concentration of 3.7 × 105 ng/L, this means that 56 ± 15 kg of identified PFASs are released into the river in addition to the 387 ± 183 kg already discharged by the WWTP.
In water used for firefighting exercises (Supplemental Table S4), the total concentration of quantified PFASs ranged from 3.6 × 105 to 6.5 × 105 ng/L and was consistent with that measured in the lagoon. The use of this water to dilute foam concentrates before training sessions slightly increases the PFAS burden released into the environment. The relative contribution of PFOS to the total PFSA concentration was similar to that observed in the lagoon, the wastewater drained from the firefighters’ exercise areas, and the runoff water (Supplemental Fig. S5).
Assuming that PFOS is a signature of PFOS-based foams and that 6:2 FTSA is a signature of FT-based foams, PFOS/6:2 FTSA ratios were calculated in every sample (Supplemental Tables S4, S5, and S7). They were systematically below 2 except in six runoff water samples, in which they ranged from 2.52 (RW-2) to 7.98 (RW-14). Such a ratio above 5 was reported in stream and groundwater from two fire drill areas where PFOS-based foams were used 6–7 years before samples were collected (SFT 2008). The runoff water samples taken in this study provide the most inclusive information on the type of foams that have been used on this site since 1987. The sampling points with the highest PFOS/6:2 FTSA ratios (RW-14, RW-1, and RW-10) are probably located near areas where PFOS-based foams were mainly used. Because RW-10 and RW-14 are two areas where no training exercises took place during the past decade (the total PFAS concentrations were some of the lowest recorded), we can assume that the PFOS predominance stems from former spills. Conversely, RW-1 was located close to an area currently used for training sessions (total PFAS concentration of 2.9 × 107 ng/L), suggesting that PFOS-based foams have been used recently.
Conclusions
PFAS contamination of soil and groundwater in the vicinity of firefighter training areas is now a well-established fact. The present study emphasizes that the runoff water and wastewater of these sites carries high concentrations of PFASs and spreads these chemicals even more broadly in the environment.
In this study, PFCAs with more than seven perfluorinated carbons (PFOAs) were only quantified in wastewater drained from the firefighters’ exercise areas and runoff water samples with the highest total PFAS concentrations (RW-1, RW-15, and RW-16). Previously published studies have also shown that water samples collected close to fire drill areas contain long-chain PFCAs, such as PFNA, PFDA, and PFUnDA (Kärrman et al. 2011). It is likely that these PFCAs are not intentionally added to some foams but are present as impurities. Because they are highly bioaccumulative, this may explain why elevated PFDA concentrations were recorded in a firefighter group compared to a representative US population group (Dobraca et al. 2015).
We should bear in mind that some major fluorinated ingredients of foams were not quantified in this study due to the lack of available standards. This means that the environmental burden of PFAS resulting from this activity was underestimated. As previously stressed (Blum et al. 2015; Scheringer et al. 2014), scientists need a truly effective collaboration with certain industries, not just words. To assess accurately the risk for environment and humans, there is an urgent need of standards for the PFASs detected in the suspect screenings and published in the literature. Furthermore, the other additives contained in foam formulations (corrosion inhibitors, anti-freezing agents, and hydrocarbon surfactants) and the leftovers of flammable liquids used for fire drills also play a part in the introduction of chemicals into the environment.
References
2006/122/EC (2006) Regulations and administrative provisions of the member states relating to restrictions on the marketing and use of certain dangerous substances and preparations (perfluorooctane sulfonates). In: Council EPaT (ed)
Anderson RH, Long GC, Porter RC, Anderson JK (2016) Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: field-validation of critical fate and transport properties. Chemosphere 150(Supplement C):678–685. https://doi.org/10.1016/j.chemosphere.2016.01.014
Arias EV, Mallavarapu M, Naidu R (2014) Identification of the source of PFOS and PFOA contamination at a military air base site. Environ Monit Assess 187(1):1–8. https://doi.org/10.1007/s10661-014-4111-0
Arvaniti OS, Andersen HR, Thomaidis NS, Stasinakis AS (2014) Sorption of perfluorinated compounds onto different types of sewage sludge and assessment of its importance during wastewater treatment. Chemosphere 111:405–411. https://doi.org/10.1016/j.chemosphere.2014.03.087
Bach C, Dauchy X, Boiteux V et al (2017) The impact of two fluoropolymer manufacturing facilities on downstream contamination of a river and drinking water resources with per- and polyfluoroalkyl substances. Environ Sci Pollut Res Int 24(5):4916–4925. https://doi.org/10.1007/s11356-016-8243-3
Backe WJ, Day TC, Field JA (2013) Zwitterionic, cationic, and anionic fluorinated chemicals in aqueous film forming foam formulations and groundwater from U.S. military bases by nonaqueous large-volume injection HPLC-MS/MS. Environ Sci Technol 47(10):5226–5234. https://doi.org/10.1021/es3034999
Baduel C, Paxman CJ, Mueller JF (2015) Perfluoroalkyl substances in a firefighting training ground (FTG), distribution and potential future release. J Hazard Mater 296:46–53. https://doi.org/10.1016/j.jhazmat.2015.03.007
Baduel C, Mueller JF, Rotander A, Corfield J, Gomez-Ramos M-J (2017) Discovery of novel per- and polyfluoroalkyl substances (PFASs) at a fire fighting training ground and preliminary investigation of their fate and mobility. Chemosphere 185(Supplement C):1030–1038. https://doi.org/10.1016/j.chemosphere.2017.06.096
Barzen-Hanson KA, Roberts SC, Choyke S et al (2017) Discovery of 40 classes of per- and polyfluoroalkyl substances in historical aqueous film-forming foams (AFFFs) and AFFF-impacted groundwater. Environ Sci Technol 51(4):2047–2057. https://doi.org/10.1021/acs.est.6b05843
Bhavsar SP, Fowler C, Day S et al (2016) High levels, partitioning and fish consumption based water guidelines of perfluoroalkyl acids downstream of a former firefighting training facility in Canada. Environ Int 94:415–423. https://doi.org/10.1016/j.envint.2016.05.023
Blum A, Balan SA, Scheringer M et al (2015) The madrid statement on poly- and perfluoroalkyl substances (PFASs). Environ Health Perspect 123(5):A107–A111. https://doi.org/10.1289/ehp.1509934
Boiteux V, Bach C, Sagres V et al (2016) Analysis of 29 per- and polyfluorinated compounds in water, sediment, soil and sludge by liquid chromatography–tandem mass spectrometry. Int J Environ Anal Chem 96(8):705–728. https://doi.org/10.1080/03067319.2016.1196683
Buck RC, Franklin J, Berger U et al (2011) Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7(4):513–541. https://doi.org/10.1002/ieam.258
Dauchy X, Boiteux V, Bach C et al (2017a) Mass flows and fate of per- and polyfluoroalkyl substances (PFASs) in the wastewater treatment plant of a fluorochemical manufacturing facility. Sci Total Environ 576:549–558. https://doi.org/10.1016/j.scitotenv.2016.10.130
Dauchy X, Boiteux V, Bach C, Rosin C, Munoz J-F (2017b) Per- and polyfluoroalkyl substances in firefighting foam concentrates and water samples collected near sites impacted by the use of these foams. Chemosphere 183:53–61. https://doi.org/10.1016/j.chemosphere.2017.05.056
Dauchy X, Boiteux V, Colin A et al (2019) Deep seepage of per- and polyfluoroalkyl substances through the soil of a firefighter training site and subsequent groundwater contamination. Chemosphere 214:729–737. https://doi.org/10.1016/j.chemosphere.2018.10.003
de Vries P, Slijkerman DME, Kwadijk CJAF et al (2017) The toxic exposure of flamingos to per- and polyfluoroalkyl substances (PFAS) from firefighting foam applications in Bonaire. Mar Pollut Bull. https://doi.org/10.1016/j.marpolbul.2017.07.017
Dobraca D, Israel L, McNeel S et al (2015) Biomonitoring in California firefighters: metals and perfluorinated chemicals. J Occup Environ Med 57(1):88–97. https://doi.org/10.1097/jom.0000000000000307
Filipovic M, Woldegiorgis A, Norström K, Bibi M, Lindberg M, Österås A-H (2015) Historical usage of aqueous film forming foam: a case study of the widespread distribution of perfluoroalkyl acids from a military airport to groundwater, lakes, soils and fish. Chemosphere 129:39–45. https://doi.org/10.1016/j.chemosphere.2014.09.005
Gewurtz SB, Bhavsar SP, Petro S et al (2014) High levels of perfluoroalkyl acids in sport fish species downstream of a firefighting training facility at Hamilton International Airport, Ontario, Canada. Environ Int 67:1–11. https://doi.org/10.1016/j.envint.2014.02.005
Houtz EF, Higgins CP, Field JA, Sedlak DL (2013) Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environ Sci Technol 47(15):8187–8195. https://doi.org/10.1021/es4018877
Kärrman A, Elgh-Dalgren K, Lafossas C, Moskeland T (2011) Environmental levels and distribution of structural isomers of perfluoroalkyl acids after aqueous fire-fighting foam (AFFF) contamination. Environ Chem 8(4):372–380
Kissa E (2001) Fluorinated surfactants and repellents, vol 97. Marcel Dekker, Inc., New York
Kwadijk CJAF, Kotterman M, Koelmans AA (2014) Partitioning of perfluorooctanesulfonate and perfluorohexanesulfonate in the aquatic environment after an accidental release of aqueous film forming foam at Schiphol Amsterdam Airport. Environ Toxicol Chem 33(8):1761–1765. https://doi.org/10.1002/etc.2602
McGuire ME, Schaefer C, Richards T et al (2014) Evidence of remediation-induced alteration of subsurface poly- and perfluoroalkyl substance distribution at a former firefighter training area. Environ Sci Technol 48(12):6644–6652. https://doi.org/10.1021/es5006187
Moody CA, Field JA (2000) Perfluorinated surfactants and the environmental implications of their use in fire-fighting foams. Environ Sci Technol 34(18):3864–3870. https://doi.org/10.1021/es991359u
Moody CA, Martin JW, Kwan WC, Muir DCG, Mabury SA (2002) Monitoring perfluorinated surfactants in biota and surface water samples following an accidental release of fire-fighting foam into Etobicoke Creek. Environ Sci Technol 36(4):545–551. https://doi.org/10.1021/es011001+
Oakes KD, Benskin JP, Martin JW et al (2010) Biomonitoring of perfluorochemicals and toxicity to the downstream fish community of Etobicoke Creek following deployment of aqueous film-forming foam. Aquat Toxicol 98(2):120–129. https://doi.org/10.1016/j.aquatox.2010.02.005
Paul AG, Jones KC, Sweetman AJ (2009) A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environ Sci Technol 43(2):386–392. https://doi.org/10.1021/es802216n
Place BJ, Field JA (2012) Identification of novel fluorochemicals in aqueous film-forming foams used by the US military. Environ Sci Technol 46(13):7120–7127. https://doi.org/10.1021/es301465n
Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH (2006) Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol 40(1):32–44. https://doi.org/10.1021/es0512475
Renner R (2001) Growing concern over perfluorinated chemicals. Environ Sci Technol 35(7):154A–160A. https://doi.org/10.1021/es012317k
Scheffey JL, Hanauska CP (2002) Status report on environmental concerns related to aqueous film forming foam (AFFF), pp 1–13
Scheringer M, Trier X, Cousins IT et al (2014) Helsingør statement on poly- and perfluorinated alkyl substances (PFASs). Chemosphere 114:337–339. https://doi.org/10.1016/j.chemosphere.2014.05.044
Schultz MM, Barofsky DF, Field JA (2004) Quantitative determination of fluorotelomer sulfonates in groundwater by LC MS/MS. Environ Sci Technol 38(6):1828–1835. https://doi.org/10.1021/es035031j
SFT (2008) Screening of polyfluorinated organic compounds at four fire training facilities in Norway. Norvegian Pollution Control Authority, Oslo, pp 1–88
Taniyasu S, Yamashita N, Yamazaki E et al (2015) Contamination profiles of perfluorinated chemicals in the inland and coastal waters of Japan following the use of fire-fighting foams. In: American Chemical Society symposium series, vol 1206, pp 221–244
Weber R, Bantz I, Klumbies M, Valentin I, Fantke P (2010) PFOS and PFC pollution from use of fire fighting foam in a major fire in Düsseldorf/Germany—human exposure and regulatory actions. Organohalogen Compd 72:1005–1008
Weber AK, Barber LB, LeBlanc DR, Sunderland EM, Vecitis CD (2017) Geochemical and hydrologic factors controlling subsurface transport of poly- and perfluoroalkyl substances, Cape Cod, Massachusetts. Environ Sci Technol 51(8):4269–4279. https://doi.org/10.1021/acs.est.6b05573
Acknowledgements
The authors thank the managers of the firefighter training site and the French Ministry of Health (DGS 216) for supporting this study. We are grateful to Delphine Libby-Claybrough for helpful insights on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dauchy, X., Boiteux, V., Colin, A. et al. Poly- and Perfluoroalkyl Substances in Runoff Water and Wastewater Sampled at a Firefighter Training Area. Arch Environ Contam Toxicol 76, 206–215 (2019). https://doi.org/10.1007/s00244-018-0585-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-018-0585-z