Abstract
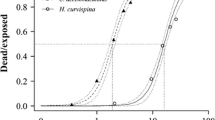
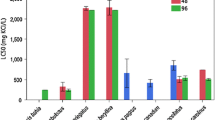
Endosulfan sulfate is a persistent environmental metabolite of endosulfan, an organochlorine insecticide–acaricide presently registered by the United States Environmental Protection Agency. There is, however, limited acute fish toxicity data for endosulfan sulfate. This study determines the acute toxicity (LC50s and LC10s) of endosulfan sulfate to three inland Florida native fish species (mosquitofish [Gambusia affinis]; least killifish [Heterandria formosa]; and sailfin mollies [Poecilia latipinna]) as well as fathead minnows (Pimephales promelas). Ninety-six-h acute toxicity tests were conducted with each fish species under flow-through conditions. For all of the above-mentioned fish species, 96-h LC50 estimates ranged from 2.1 to 3.5 μg/L endosulfan sulfate. The 96-h LC10 estimates ranged from 0.8 to 2.1 μg/L endosulfan sulfate. Of all of the fish tested, the least killifish appeared to be the most sensitive to endosulfan sulfate exposure. The above-mentioned data were combined with previous acute toxicity data for endosulfan sulfate and freshwater fish for an effects analysis. The effects analysis estimated hazardous concentrations expected to exceed 5, 10, and 50% of the fish species’ acute LC50 or LC10 values (HC5, HC10, and HC50). The endosulfan sulfate freshwater-fish acute tests were also compared with the available freshwater-fish acute toxicity data for technical endosulfan. Technical endosulfan is a mixture of α- and β-endosulfan. The LC50s had a wider range for technical endosulfan, and their distribution produced a lower HC10 than for endosulfan sulfate. The number of freshwater-fish LC50s for endosulfan sulfate is much smaller than the number available for technical endosulfan, reflecting priorities in examining the toxicity of the parent compounds of pesticides. The toxicity test results and effects analyses provided acute effect values for endosulfan sulfate and freshwater fish that might be applied in future screening level ecologic risk assessments. The effects analyses also discussed several deficiencies in conventional methods for setting water-quality criteria and determining ecologic effects from acute toxicity tests.


Similar content being viewed by others
References
Aquatic Effects Dialogue Group (1994) Aquatic effects dialogue group: pesticide risk assessment and mitigation. Society of Environmental Toxicology and Chemistry and SETAC Foundation for Environmental Education, Pensacola, FL
Awasthi N, Ahuja R, Kumar A (2000) Factors influencing the degradation of soil-applied endosulfan isomers. Soil Biol Biochem 32:1697–1705
Berrill M, Coulson D, McGillivray Lise, Pauli B (1998) Toxicity of endosulfan to aquatic stages of anuran amphibians. Environ Toxicol Chem 17:1738–1744
Carriger JF, Rand GM (2008) Aquatic risk assessment of pesticides in surface waters in and adjacent to the Everglades and Biscayne National Parks: II. Probabilistic analyses. Ecotoxicology 17:680–696
Carriger JF, Hoang TC, Rand GM (2010) Survival analysis of mosquitofish (Gambusia affinis) and least killifish (Heterandria formosa) exposed to endosulfan sulfate. Arch Environ Contam Toxicol 58:1015–1022
Cotham WE Jr, Bidleman TF (1989) Degradation of malathion, endosulfan, and fenvalerate in seawater and seawater/sediment microcosms. J Agric Food Chem 37:824–828
Crane M, Grosso A (2002) Time to event analysis of standard ecotoxicity data. In: Crane M, Newman MC, Chapman PF, Fenlon J (eds) Risk assessment with time to event models. Lewis, CRC Press, Boca Raton, FL
De Zwart D (2002) Observed regularities in SSDs for aquatic species. In: Posthuma L, Suter GW II, Traas TP (eds) Species sensitivity distributions in ecotoxicology. Lewis, CRC Press, Boca Raton, FL
ECOFRAM (1999) ECOFRAM aquatic report. Ecological committee on FIFRA risk assessment methods: report of the aquatic workgroup. United States Environmental Protection Agency, Office of Pesticide Programs, Washington, DC
Fox D (2008) NECS, NOECS and the EC. Australas J Ecotoxicol 14:7–9
Fox D (2009) Is the ECX a legitimate surrogate for a NOEC. Integr Environ Assess Manage 5:351–353
German Federal Environment Agency (2007) Endosulfan: draft dossier prepared in support of a proposal of endosulfan to be considered as a candidate for inclusion in the Annexes to the Stockholm Convention. Umweltbundesamt, Dessau
Haider S, Imbaray RM (1986) Relative toxicity of technical material and commercial formulation of malathion and endosulfan to a fresh water fish, Channa punctata. Ecotoxicol Environ Safe 11:347–351
Hose GC, Van den Brink PJ (2004) Confirming the species-sensitivity distribution concept for endosulfan using laboratory, mesocosm, and field data. Arch Environ Contam Toxicol 47:511–520
Hose GC, Hyne RV, Lim RP (2003) Toxicity of endosulfan to Atalophlebia spp. (Ephemeroptera) in the laboratory, mesocosm, and field. Environ Toxicol Chem 22:3062–3068
Knauf W, Schulze EF (1973) New findings on the toxicity of endosulfan and its metabolites to aquatic organisms. Meded Fac Landbouwwetensch. Rijksuniv Gent 38:717–732
Landis WG, Yu MH (2004) Introduction to environmental toxicology: impacts of chemicals upon ecological systems, 3rd edn. Lewis, CRC Press, Boca Raton, FL
Lemke AE (1980) Interlaboratory comparison acute testing set. Comprehensive report. In: Ambient water quality criteria. EPA 440/5-80-046. United States Environmental Protection Agency, Washington, DC, pp B-1–B-35
Leonard AW, Hyne RV, Lim RP, Pablo F, Van den Brink PJ (2000) Riverine endosulfan concentrations in the Namoi River, Australia: link to cotton field runoff and macroinvertebrate population densities. Environ Toxicol Chem 19(6):1540–1551
Martens R (1976) Degradation of [8,9-14C] endosulfan by soil microorganisms. Appl Environ Microbiol 31(6):853–858
Martens R (1977) Degradation of endosulfan-8, 9-14C in soil under different conditions. Bull Environ Contam Toxicol 17(4):438–446
Miles JRW, Moy P (1979) Degradation of endosulfan and its metabolites by a mixed culture of soil microorganisms. Bull Environ Contam Toxicol 23:13–19
Moore DRJ, Caux PY (1997) Estimating low toxic effects. Environ Toxicol Chem 16:794–801
Mount DI, Brungs WA (1967) A simplified dosing apparatus for fish toxicology studies. Wat Res 1:21–29
Nebeker AV, McCrady JK, Mshar R, McAuliffe CK (1983) Relative sensitivity of Daphnia magna, rainbow trout and fathead minnows to endosulfan. Environ Toxicol Chem 1:69–72
Newman MC (1995) Quantitative methods in aquatic ecotoxicology. Lewis, Boca Raton, FL
Newman MC (2008) What exactly are you inferring?―A closer look at hypothesis testing. Environ Toxicol Chem 27:1013–1019
Norberg-King T (1993) A linear interpolation method for sublethal toxicity: the inhibition concentration (ICp) approach (version 2.0). National Effluent Toxicity Assessment Center Technical Report 03-93, United States Environmental Protection Agency, Environmental Research Laboratory, Duluth, MN
Pfeuffer R, Matson F (1998–2007) Pesticide surface water report. Quarterly sampling event. South Florida Water Management District, West Palm Beach, FL
Rand GM, Carriger JF, Gardinali PR, Castro J (2010) Endosulfan and its metabolite, endosulfan sulfate, in freshwater ecosystems of south Florida: a probabilistic aquatic ecological risk assessment. Ecotoxicology 19:879–900
Rao DMR, Murty AS (1980) Persistence of endosulfan in soils. J Agric Food Chem 28:1099–1101
Rodney S, Moore D (2008) Development of an excel-based tool for fitting and evaluating species sensitivity distributions: final report. Intrinsik Science, Ottawa, Ontario
Sericano JL, Gardinali PR, Wade TL (1998) Quantitative determination of chlorinated hydrocarbons. In: Lauenstein GG, Cantillo AY (eds) Sampling and analytical methods of the national status and Trends Program Mussel Watch Project: 1993–1996 Update. NOAA Technical Memorandum NOS ORCA 130. National Oceanic and Atmospheric Administration (NOAA), National Ocean Service, Silver Spring, MD, pp 160–167
Software Tidepool Scientific (2004) ToxCalc: toxicity data analysis software v5.0.26. Tideppol Scientific Software, McKinleyville, CA
Stewart DKR, Cairns KG (1974) Endosulfan persistence in soil and uptake by potato tubers. J Agric Food Chem 22(6):984–986
Sunderam RIM, Cheng DMH, Thompson GB (1992) Toxicity of endosulfan to native and introduced fish in Australia. Environ Toxicol Chem 11:1469–1476
Unger MA, Newman MC, Vadas GG (2007) Predicting survival of grass shrimp (Palaemnetes pugio) during ethylnaphthalene, dimethylnaphthaalene, and phenanthrene exposures differing in concentration and duration. Environ Toxicol Chem 26:528–534
United States Environmental Protection Agency (2002a) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. EPA-821-R-02-012. United States Environmental Protection Agency, Washington, DC
United States Environmental Protection Agency (2002b) Reregistration eligibility decision for endosulfan. EPA 738-R-02-013, United States Environmental Protection Agency, Prevention, Pesticides and Toxic Substances, Washington, DC
United States Environmental Protection Agency (2002c) Reregistration eligibility science chapter for atrazine: environmental fate and effects chapter. Appendix XV. Comparison of OW and OPP methods for aquatic assessments. United States Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Washington, DC
United States Environmental Protection Agency (2007) Appendix 1 to 2007 addendum: environmental fate and ecological risk assessment of endosulfan. United States Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances, Washington, DC
Van Dyk LP, Van der Linde A (1976) Persistence of endosulfan in soils of the Loskop Dam irrigation area. Agrochemophysica 8:31–34
Wan MT, Kuo J, Buday C, Schroeder G, Van Aggelen G, Pasternak J (2005) Toxicity of α-, β-, (α + β)-endosulfan and their formulated and degradation products to Daphnia magna, Hyalella azteca, Oncorhynchus mykiss, Oncorhynchuc kisutch, and biological implications in streams. Environ Toxicol Chem 24:1146–1154
You J, Schuler LJ, Lydy MJ (2004) Acute toxicity of sediment-sorbed endrin, methoxychlo and endosulfan to Hyalella azteca and Chironomus tentans. Bull Environ Contam Toxicol 73:457–464
Acknowledgments
This study was funded by the Everglades National Park through cooperative agreement number H5297-04-0133. We thank Dwayne Moore of Intrinsik Environmental Sciences, Inc. for providing us with a copy of SSD Master. This is SERC contribution number 499.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carriger, J.F., Hoang, T.C., Rand, G.M. et al. Acute Toxicity and Effects Analysis of Endosulfan Sulfate to Freshwater Fish Species. Arch Environ Contam Toxicol 60, 281–289 (2011). https://doi.org/10.1007/s00244-010-9623-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-010-9623-1