Abstract
To determine the potential impact of contaminants on the aquatic vascular plants Lemna sp., toxicity tests are usually conducted for a 4- to 14-day exposure, and the toxicity is usually expressed as EC50. However, the effects of longer exposure and the recovery potential after exposure to chemicals are other important factors which should be considered. We present the relative risks of a variety of exposure scenarios and recovery potentials from damage, using herbicides with different modes of action. Toxicity was assessed on the basis of both EC50 and relative growth rate (RGR) compared with untreated controls in exposure and recovery. The EC50 of atrazine was found to be 89 ppb, and its phytostatic concentrations were 1600 and 800 ppb for xposure periods of 14 and 28 days, respectively, and no phytocidal effects were observed up to 3200 ppb for a 28-day exposure. The RGR in recovery was not affected by the RGR in exposure, and regrowth was possible even after complete inhibition of growth for 28 days at the highest concentration tested. Alachlor, with an EC50 of 31 ppb, was phytostatic at 400 ppb for a 14-day exposure and phytocidal at 200 ppb for 21- and 28-day exposures. Paraquat, with an EC50 of 31 ppb, showed phytocidal rather than phytostatic effects. All phytostatic fronds could not grow in the recovery period, and the phytocidal concentration decreased with exposure period, from 80 ppb for a 7-day exposure to 20 ppb for 21- and 28-day exposures. The RGR of alachlor and paraquat in recovery was dependent on the RGR in exposure. In the case of cyclosulfamuron, phytostatic concentrations were 100 and 50 ppb for 7- and 14-day exposures, respectively. In the case of exposures longer than 21 days, however, it exhibited phytocidal activity at 10 ppb. The results of this study suggest that it is important to examine the effects of chemicals over a longer exposure period as well as the recovery potential from damage for reliable ecological risk assessment.


Similar content being viewed by others
References
American Society for Testing and Mateials (1993) Standard guide for conducting static toxicity tests with Lemna gibba G3. Annual book of ASTM standards, section 11, vol 11.04. Designation E1415-91. ASTM, Philadelphia, pp 1137–1146
Corre G, Templier J, Largeau C (1996) Influence of cell wall composition on the resistance of two Chlorella species (Chlorophyta) to detergents. J Phycol 32:584–590
Couderchet M, Boger P (1993) Chloroacetamide-induced reduction of fatty acid desaturation. Pestic Biochem Physiol 45:91–97
Davies J, Honegger JL, Tencalla FG, Meregalli G, Brain P, Newman JR, Pitchford HF (2003) Herbicide risk assessment for non-target aquatic plants: sulfosulfuron—a case study. Pest Manage Sci 59:231–237
Eisler R (1990) Paraquat hazards to fish, wildlife and invertebrates: a synoptic review. Contaminant Hazard Reviews. Biological Report 85 (1.22). U.S. Fish Wildlife Service,
Fairchild JF, Ruessler DS, Haverland PS, Carlson AR (1997) Comparative sensitivity of Selenastrum capricornutum and Lemna minor to sixteen herbicides. Arch Environ Contam Toxicol 32:353–357
Grace JB, Wetzel RG (1978) The production biology of eurasian watermilfoil (Myriophyllum spicatum L.): a review. J Aquat Plant Manage 16:1–11
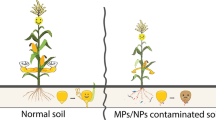
He ZL, Yanga XE, Stoffellab PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol 19:125–140
Hoberg JR (1991) Atrazine technical-toxicity to duckweed (Lemna gibba G3). SLI Report 91–1-3613. Springborn Laboratories, Wareham, MA
Hoberg JR (1993a) Atrazine technical-toxicity to duckweed (Lemna gibba G3). SLI Report 93–11-5053. Springborn Laboratories, wareham, MA
Hoberg JR (1993b) Atrazine technical-toxicity to duckweed (Lemna gibba G3). SLI Report 93–4-4755. Springborn Laboratories, Wareham, MA
Hughes JS, Alexander MM, Balu K (1988) An evaluation of appropriate expressions of toxicity in Aquatic plant bioassays as demonstrated by the effects of atrazine on algae duckweed. Aquatic Toxicology and Hazard Assessment, vol 10. American Society for Testing and Materials, Philadelphia, pp 531–547
Kloeppel H, Koerdel W, Stein B (1997) Herbicide transport by surface runoff and herbicide retention in a filter strip rainfall and runoff simulation studies. Chemosphere 35:129
Lewis MA (1995) Use of freshwater plants for phytotoxicity testing: a review. Environ Pollut 87:319–336
McKelvey RA, Wrigh JP, Honegger J (2002) A comparison of crop and non-crop plants as sensitive indicator species of regulatory testing. Pest Manage Sci 58:1161–1174
Moberg WK, Cross B (1990) Herbicides inhibiting branched-chain amino acid biosynthesis. Pest Sci 29:241–246
Mohammad M, Kishimoto T, Itoh K, Suyama K, Yamamoto H (2005) Comparative sensitivity of Pseudokirchnetiella subcapitata vs. Lemna sp. to eight sulfonylurea herbicides. Bull Environ Contam Toxicol 75:866–872
Mohammad M, Itoh K, Suyama K, Yamamoto H (2006) Recovery of Lemna sp. after exposure to sulfonylurea herbicides. Bull Environ Contam Toxicol 76:256–263
Mohammad M, Itoh K, Suyama K (2008) Comparative effects of different families of herbicides on recovery potentials in Lemna sp. J Pestic Sci 33:171–174
CD OE (2006) OECD guidelines for the testing of chemicals, revised proposal for a new guideline 221, Lemna sp. growth inhibition test. OECD, Paris
Peterson HG, Boutin C, Martin PA, Freemark KE, Ruecker NJ, Moody MJ (1994) Aquatic phyto-toxicity of 23 pesticides applied at expected environmental concentrations. Aquat Toxicol 28:275–292
Pothuluri JV, Moorman TB (1990) Aerobic and anaerobic degradation of alachlor in samples from a surface-to-groundwater profile. J Environ Qual 19:525–530
Rao PSC, Davidson JM (1980) Estimation of pesticide retention and transformation parameters required in nonpoint source pollution models. In: Overcash MR, Davidson JM (eds) Environmental impact of nonpoint source pollution. Ann Arbor Science, Ann Arbor, MI
Saenz ME, Di MarzioWD, Alberdi JL, Tortorelli MC (2001) Algal growth recovery studies after paraquat exposure. Bull Environ Contam Toxicol 66:263–268
Spawn RL, Kyle DH, Blair DS (1997) Effects of alachlor on an algal community from a midwestern agricultural stream. Environ Toxicol Chem 16:785–793
Tomlin CDS (ed) (2000) The pesticide manual, 12th edn. British Crop Protection Council, UK
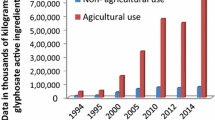
USDA (1998) Agicultural chemical usage: field crop summary. U.S. Department of Agriculture, Washington, DC
U.S. Environmental Protection Agency (1987) Paraquat-health advisory. Office of Drinking Water, Washington, DC
U.S. Environmental Protection Agency (1988) Atrazine-health advisory. Office of Drinking Water, Washington, DC, p 861
U.S. Environmental Protection Agency (1996) OPPTS 850.4400 Aquatic plant toxicity test using Lemna sp., tiers I and II. EPA 712-C-96–156. EPA, Washington, DC
U.S. Environmental Protection Agency (1998) Risk Assessment Forum. Guidelines for ecological risk assessment. EPA/630/R-95/002F. EPA, Washington, DC
Wang W (1990) Review on duckweed toxicity testing. Environ Res 52:7–22
Weisshaar H, Retzlaff G, Boger P (1993) Chloroacetamide-induced reduction of fatty acid synthesis. Pestic Biochem Physiol 32:212–216
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammad, M., Itoh, K. & Suyama, K. Effects of Herbicides on Lemna gibba and Recovery from Damage After Prolonged Exposure. Arch Environ Contam Toxicol 58, 605–612 (2010). https://doi.org/10.1007/s00244-010-9466-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-010-9466-9