Abstract
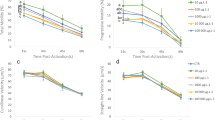
Many toxic effects of treated wastewater effluent on organismal and reproductive health have been documented. However, the physicochemical environment of treated wastewater effluent frequently differs considerably from that of its receiving waters and may affect organismal function independently of toxic effects. Teleost sperm, for example, may be affected by the higher osmolality of treated wastewater, as this sperm is activated for a brief period of time following ejaculation due to the sudden decrease in osmolality of its surrounding environment. In this study, we examined the effects of treated wastewater effluent on sperm motility to test the hypothesis that the higher osmolality of effluent compared to river water will adversely affect sperm activation in a concentration-dependent relationship. Treated wastewater effluent was collected on 5 days from the outflow of the Metropolitan Wastewater Treatment Plant, St. Paul, Minnesota, and from an upstream site on the Mississippi River. Milt aliquots collected from goldfish were diluted in an isotonic extender solution and subsequently activated in either deionized water, 100%, 50%, or 10% effluent, a synthetic ion mixture, or river water. Sperm motility and velocity were assessed at 15-s intervals for 1 min using a computer assisted sperm analyzer. Significant differences in performance parameters were found only at 15 s, with sperm motility and velocity declining rapidly at later sampling times. Predictably, deionized water resulted in the greatest activation of sperm motility, while motility exhibited a concentration-dependent decline in 10%, 50%, and 100% treated wastewater effluent. Interestingly, Mississippi River water and a synthetic ion mixture with an osmolality comparable to 50% effluent both resulted in the least amount of sperm activation. However, sperm activation in river water varied between collection days during the study. River water and 100% effluent both had low sperm activation characteristics despite a 10-fold difference in osmolality between these two treatments (1 and 10 mOsmol kg−1, respectively). Results of this study indicate a concentration-dependent decrease in sperm motility in treated wastewater effluent as well as significant fluctuations of sperm activation in Mississippi River water. This study illustrates the complexity of assessing the effects of treated wastewater effluents and the difficulty of determining appropriate reference sites for such studies.





Similar content being viewed by others
References
Adeoya-Osiguwa SA, Markoulaki S, Pocock V, Milligan SR, Fraser LR (2003) 17ß estradiol and environmental estrogens significantly affect mammalian sperm function. Human Reprod 18:100–107. doi:10.1093/humrep/deg037
Alavi SMH, Cosson J (2005) Sperm motility in fishes. I. Effects of temperature and pH: a review. Cell Biol Int 29:101–110. doi:10.1016/j.cellbi.2004.11.021
Alavi SMH, Rodina M, Policar T, Jozak P, Psenicka M, Linhart O (2007) Semen of Perca fluviatilis L.: sperm volume and density, seminal plasma indices and effects of dilution ratio, ions and osmolality on sperm motility. Theriogenology 68:276–283. doi:10.1016/j.theriogenology.2007.05.045
Astanin LP, Podgornyy MI (1968) Features of the fertility of Carassius carassius (L.) and Carassius auratus gibelio (Bloch). J Ichthyol 8:209–214
Barber LB, Lee KE, Swackhamer DL, Schoenfuss HL (2007) Reproductive responses of male fathead minnows exposed to wastewater treatment plant effluent, effluent treated with XAD8 resin, and an environmentally relevant mixture of alkylphenol compounds. Aquat Toxicol 82:36–46. doi:10.1016/j.aquatox.2007.01.003
Billard R (1978) Changes in structure and fertilization ability of marine and freshwater fish spermatozoa diluted in media of various salinities. Aquaculture 14:187–198. doi:10.1016/0044-8486(78)90094-7
Billard R, Cosson J, Perchec G, Linhart O (1995) Biology of sperm and artificial reproduction in carp. Aquaculture 129:95–112. doi:10.1016/0044-8486(94)00231-C
Carroll AM, Wainwright PC, Huskey SH, Collar DC, Turigan RG (2004) Morphology predicts suction feeding performance in centrachid fishes. J Exp Biol 207:3873–3881. doi:10.1242/jeb.01227
Chao N, Chao W, Liu K, Liao I (1987) The properties of tilapia sperm and its cryopreservation. J Fish Biol 30:107–118. doi:10.1111/j.1095-8649.1987.tb05737.x
Christ S, Toth G, McCarthy H, Torsella J, Smith M (1996) Monthly variation in sperm motility in common carp assessed using computer-assisted sperm analysis (CASA). J Fish Biol 48:1210–1222. doi:10.1111/j.1095-8649.1996.tb01815.x
Coutant C (1970) Biological aspects of thermal pollution. I. Entrainment and discharge canal effects. Rev Environ Control 1:342–381
DeFraipont M, Sorensen PW (1993) Exposure to the pheromone 17a, 20b-dihydroxy-4-pregnen-3-one enhances the behavioral spawning success, sperm production and sperm motility of male goldfish. Anim Behav 46:245–256. doi:10.1006/anbe.1993.1186
Desbrow C, Routledge E, Brighty G, Sumpter J, Waldock M (1998) Identification of estrogenic chemicals in STW effluent. I. Chemical fractionation and in vitro biological screening. Environ Sci Technol 32:1549–1558. doi:10.1021/es9707973
Dzuba B, Bozhok G, Rudenko S (2001) A study of the dynamics of volume change during the period of active motility in carp, Cyprinus carpio L., spermatozoa. Aquacult Res 31:51–56. doi:10.1046/j.1365-2109.2001.00527.x
Farley CT (1997) Maximum speed and mechanical power output in lizards. J Exp Biol 200:2189–2195
Fassel VA, Kniseley RN (1974) Inductively coupled plasma optical emission spectroscopy. Anal Chem 46:1110A–1120A. doi:10.1021/ac60349a023
Folmar L, Denslow N, Rao V, Chow M, Crain A, Enblom J, Marcino J, Guillette L Jr (1996) Vitellogenin introduction and reduced serum testosterone concentrations in feral male carp (Cyprinus carpio) captured near a major metropolitan wastewater treatment plant. Environ Health Perspect 104:1096–1100. doi:10.2307/3433123
Folmar L, Denslow N, Kroll K, Orlando E, Enblom J, Marcino J, Metcalfe C, Guilette L Jr (2001) Altered serum sex steroids and vitellogenin induction in walleye (Stizostedion vitreum) collected near a metropolitan wastewater treatment plant. Arch Environ Contam Toxicol 40:392–398. doi:10.1007/s002440010188
Full RJ, Yamauchi A, Jindrich DL (1995) Maximum single leg force production: cockroaches righting on photoelastic gelatin. J Exp Biol 198:2441–2452
Jin W, Arai KY, Watanabe G, Suzuki AK, Takahashi S, Taya K (2005) The stimulatory role of estrogen on sperm motility in the male golden hamster (Mesocricetus auratus). J Androl 26:478–484. doi:10.2164/jandrol.04167
Jobling S, Nolan M, Tyler C, Brighty G, Sumpter J (1998) Widespread sexual disruption in wild fish. Environ Sci Technol 32:2498–2506. doi:10.1021/es9710870
Kime DE (1999) A strategy for assessing the effects of xenobiotics on fish reproduction. Sci Total Environ 225:3–11. doi:10.1016/S0048-9697(98)00328-3
Kime DE, Nash JP (1999) Gamete viability as an indicator of reproductive endocrine disruption in fish. Sci Total Environ 233:123–129. doi:10.1016/S0048-9697(99)00219-3
Kime DE, Ebrahimi M, Nysten K, Roelants I, Rurangwa E, Moore H, Olivier F (1996) Use of computer assisted sperm analysis (CASA) for monitoring the effects of pollution on sperm quality of fish. Application to the effects of heavy metals. Aquat Toxicol 36:223–237. doi:10.1016/S0166-445X(96)00806-5
Kime DE, Van Look K, McAllister B, Huyskens G, Rurangwa E, Ollevier F (2001) Computer-assisted sperm analysis (CASA) as a tool for monitoring sperm quality in fish. Comp Biochem Physiol C 130:425–433
Kleinkauf A, Macfarlane C, Yeates S, Simpson MG, Leah RT (2004) A biomarker approach to endocrine disruption in flounder—estrogen receptors, hepatocyte proliferation and sperm motility. Ecotoxicol Environ Safety 58:324–334. doi:10.1016/j.ecoenv.2003.10.004
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ Sci Technol 36:1202–1211. doi:10.1021/es011055j
Krasznai Z, Marian T, Balkay L, Gaspar R Jr, Tron L (1995) Potassium channels regulate hypo-osmotic schock-induced motility of common carp (Cyprinus carpio) sperm. Aquaculture 129:123–128. doi:10.1016/0044-8486(94)00234-F)
Krasznai Z, Marian T, Izumi H, Damjanovich S, Balkay L, Tron L, Morisawa M (2000) Membrane hyperpolarization removes inactivation of Ca2+ channels, leading to Ca2+ influx and subsequent initiation of sperm motility in the common carp. Proc Natl Acad Sci USA 97:2052–2057. doi:10.1073/pnas.040558097
Kyle A, Stacey N, Peter R, Billard R (1985) Elevations in gonadotrophin concentrations and milt volumes as a result of spawning behavior in the goldfish. Gen Comp Endrocrinol 57:10–22. doi:10.1016/0016-6480(85)90195-9
Lahnsteiner F, Berger B, Grubinger F, Weismann T (2005a) The effect of 4-nonylphenol on semen quality, viability of gametes, fertilization success, and embryo and larvae survival in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 71:297–306. doi:10.1016/j.aquatox.2004.11.007
Lahnsteiner F, Berger B, Kletzl M, Weismann T (2005b) Effect of bisphenol A on maturation and quality of semen and eggs in brown trout, Salmon trutta f. fario. Aquat Toxicol 75:213–224. doi:10.1016/j.aquatox.2005.08.004
Liu D, Clarke G, Gordon Baker H (1991) Relationship between sperm motility assessed with the Hamilton-Thorn motility analyzer and fertilization rates in vitro. J Androl 12:231–239
Moore H, Akhondi M (1996) Fertilizating capacity of rat spermatozoa is correlated with decline in straight-line velocity measured by continuous computer-aided sperm analysis: Epididymal rat spermatozoa from the proximal cauda have a greater fertilizing capacity in vitro than those from the distal cauda or vas deferans. J Androl 17:50–60
Morisawa M (1994) Cell signaling mechanisms for sperm motility. Zool Sci 11:647–662
Morisawa M, Suzuki K (1980) Osmolality and potassium ion: their role in initiation and sperm motility in teleosts. Science 210:1145–1147. doi:10.1126/science.7444445
Morisawa M, Suzuki K, Shimizu H, Morisawa S, Yasuda K (1983) Effects of osmolality and potassium on motility of spermatozoa from freshwater cyprinid fishes. J Exp Biol 107:95–103
Petersen C, Warner R (1998) Sperm competition in fishes. In: Birkhead TR, Moller AP (eds) Sperm competition and sexual selection. Academic Press, New York, pp 435–464
Rodgers-Gray T, Jobling S, Morris S, Kelly C, Kirby S, Janbakhsh A, Harries J, Waldock M, Sumpter J, Tyler C (2000) Long-term temporal changes in the estrogenic composition of treated wastewater effluent and its biological effects on fish. Environ Sci Technol 34:1521–1528. doi:10.1021/es991059c
Rodgers-Gray T, Jobling S, Kelly C, Morris S, Brighty G, Waldock M, Sumpter Tyler C (2001) Exposure of juvenile roach (Rutilus rutilus) to treated wastewater effluent induces dose-dependent and persistent disruption in gonadal duct development. Environ Sci Technol 35:462–470. doi:10.1021/es001225c
Runnalls T, Hala DN, Sumpter JP (2007) Preliminary studies into the effects of the human pharamecutical Clofibtric acid on sperm parameters in adult fathead minnows. Aquat Toxicol 84:111–118. doi:10.1016/j.aquatox.2007.06.005
Rurangwa E, Biegniewska A, Slominska E, Skorkowski E, Ollevier F (2002) Effect of tributyltin on adenylate content and enzyme activities of teleost sperm: a biochemical approach to study the mechanisms of toxicant reduced spermatozoa motility. Comp Biochem Physiol C 131:335–344
Schoenfuss HS, Levitt JT, Van Der Kraak GJ, Sorensen PW (2002) Ten week exposure to treated wastewater effluent discharge has relatively minor, variable effects on reproductive behavior and sperm production in goldfish. Environ Toxicol Chem 21:2185–2190. doi :10.1897/1551-5028(2002)021<2185:TWETTS>2.0.CO;2
Stacey NE, Chamberlain K, Sorensen P, Dulka J (1987) Milt volume increase in goldfish: interaction of pheromonal and behavioral stimuli. In: Proceedings of the 3rd International Symposium on Reproductive Physiology of Fish, Alberta, Canada
Stacey NE, Fraser E, Sorensen PW, Van Der Kraak G (2000) Milt production in goldfish: regulation by multiple social stimuli. Comp Biochem Physiol B 130:467–476
Stoss J (1983) Fish gamete preservation and spermatozoa physiology. In: Hoar WS, Randall DJ, Donaldson EM (eds) Fish physiology 1X B. Academic Press, New York, pp 305–350
Swanson C, Young PS, Cech JC Jr (1998) Swimming performance of delta smelt: maximum performance, and behavioral and kinematic limitations of swimming at submaximal velocities. J Exp Biol 201:333–345
Takai M, Morisawa M (1995) Change in intracellular K+ concentration caused by external osmolality change regulates sperm motility of marine and freshwater teleosts. J Cell Sci 108:1175–1181
Tan-Fermin J, Miura T, Adachi S, Yamauchi K (1999) Seminal plasma composition, sperm motility, and milt dilution in the Asian catfish Clarias macrocephalus (Gunther). Aquaculture 171:323–338. doi:10.1016/S0044-8486(98)00402-5
Ternes T (1998) Occurrence of drugs in German wastewater treatment plants and rivers. Water Res 32:3245–3260. doi:10.1016/S0043-1354(98)00099-2
Ternes T, Stumpf M, Mueller J, Haberer K, Wilken R-D, Servos M (1999) Behavior and occurrence of estrogens in municipal wastewater treatment plants. I. Investigations in Germany, Canada, and Brazil. Sci Total Environ 225:81–90. doi:10.1016/S0048-9697(98)00334-9
U.S. Environmental Protection Agency (1983) Method 200.7. Methods for chemical analysis of water and wastes. EPA-600 4–79-020. Environmental Monitoring and Support Laboratory, Office of Research and Development, Cincinnati, OH
Warnecke D, Pluta H-J (2003) Motility and fertilization capacity of frozen/thawed common carp (Cyprinus carpio L.) sperm using dimethyl-acetamide as the main cryoprotectant. Aquaculture 215:167–185. doi:10.1016/S0044-8486(02)00371-X
Wilson R, Franklin CE, James RS (2000) Allometric scaling relationships of jumping performance in the striped marsh frog, Limnodynastes peronii. J Exp Biol 203:1937–1946
Acknowledgments
The authors thank P. Sorensen for laboratory facilities, M. Troedsson (University of Minnesota) for making the computer-assisted sperm analyzer available to us, and K. Loseth for providing training on its use. Glen Parsons at the University of Mississippi determined the osmolality of the activation solutions. B. Polta provided logistical support. Two anonymous reviewers provided excellent suggestions for improving this manuscript. This work is the result of research sponsored by the Minnesota Sea Grant College Program supported by the NOAA Office of Sea Grant, U.S. Department of Commerce, under Grant NOAA-NA86-RG0033.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schoenfuss, H.L., Levitt, J.T., Rai, R. et al. Treated Wastewater Effluent Reduces Sperm Motility Along an Osmolality Gradient. Arch Environ Contam Toxicol 56, 397–407 (2009). https://doi.org/10.1007/s00244-008-9219-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-008-9219-1