Abstract
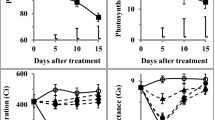
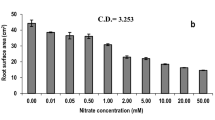
The effect of excess Ni (1 mM Ni) on wheat plants as well as the role of Ca (1 mM Ni+5000 μM Ca) for amelioration of toxicity and recovery of growth and photosynthesis in Ni-stressed wheat was evaluated. Growth, nutrient status (Ca, Mg, Fe, K, Na), and photosynthesis showed a distinct decrease strictly related to the period of treatment. Calcium ameliorated to a certain extent toxic symptoms of Ni, due to antagonistic action between Ni and Ca ions. Since chlorophyll content and variable fluorescence (Fv) decreased significantly, but Fo did not particularly change, the decrease of t1/2 with increasing duration of Ni exposure indicates negative changes on the acceptor side of PSII, which also may result from diminution of Calvin cycle. The maximum quantum yield for energy trapping was also suppressed. Plant transfer to Hoagland solution+5000 μM Ca caused recovery to plant morphology and physiology. Even in control plants, during recovery period an increased Ca concentration in plant tissues with concomitant increased rates of growth and morphology was observed. Ni concentration in plants exposed to 1 mM Ni+5000 μM Ca was lower than in plants exposed to 1 mM Ni. In all treatments a certain increase of plant nutrients was observed during recovery.





Similar content being viewed by others
References
Abadia A, Lemoine Y, Tremolieres A, Ambard-Bretteville F, Reny R (1989) Iron deficiency in pea: effects on pigment, lipid and pigment-protein complex composition of thylakoids. Plant Physiol Biochem 27:679–689
Alloway BJ (1995) Heavy metals in soils. Blackie Acad, London, pp 386
Baker NR (1991) A possible role for photosystem II in environmental perturbations of photosynthesis. Physiol Plant 81:563–570
Baron M, Arellano B, Lopez George J (1995) Copper and photosystem II: A controversial relationship. Physiol Plant 94:174–180
Dalton DA, Russel SA, Evans HJ (1988) Minireview. Nickel as a micronutrient element for plants. Biofactors 1:11–16
Epron D, Dreyer E (1993) Photosynthesis of oak leaves under water stress: maintenance of high photochemical efficiency of photosystem II and occurrence of non-uniform CO2 assimilation. Tree Physiol 13:107–117
Gabbrielli R, Gori P, Scala A (1995) Ni toxicity on carnation (Dyanthus-cariophyllus L. cv corrida) cell-cultures-selection of Ni tolerant lines and effects of Ca and Mg. Plant Sci 104:225–230
Gussarson M, Asp H, Adalsteinsson S, Jensen P (1996) Enhancement of cadmium effects on growth and nutrient composition of birch (Betula pendula) by buthionine sulphoximite (BSO). J Exp Botany 47:211–215
Hanson JB (1984) The function of calcium in plant nutrition. In : Tinker PB, Läuchli A (eds) Advances in plant nutrition. Praeger, New York, pp 149–208
Horst WJ, (1987) Aluminium tolerance and calcium efficiency of cowpea genotypes. J Plant Nutr 10:1121–1129
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants CRC Press Inc, Boca Raton, FL, USA
Kinraide TB, (1998) Three mechanisms for the calcium alleviation of mineral toxicities. Plant Physiol 118:513–520
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: The basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Krieger A, Weis E (1993) The role of calcium in the pH-dependent control of photosystem II. Photosynth Res 37:117–130
Krupa Z, Siedlecka A, Maksymiec W, Baszynski T (1993) In vivo response of photosynthetic apparatus of Phaseolus vulgaris L. to nickel toxicity. J Plant Physiol 142:664–668
Lichtenthaler HK, Rinderle U (1988) The role of chlorophyll fluorescence in the detection of stresses conditions in plants. CRC Crit Rev Anal Chem 19:29–85
Lindberg S, Wingstrand G (1985) Mechanism for Cd2+ inhibition of (K++Mg2+) ATpase activity and K+ (Rb86+) uptake join roots of sugar-beet (Beta vulgaris). Physiol Plantarum 63:181–186
Lynch J, Cramer GR, Lauchli A (1987) Salinity reduces membrane-associated calcium in corn root protoplasts. Plant Physiol 83:390–394
Maksymiec W, Baszynski T (1996) Chlorophyll fluorescence in primary leaves of excess Cu-treated runner bean plants depends on their growth stages of their primary leaves. J Plant Physiol 149:196–200
Maksymiec W, Baszynski T (1999) The role of Ca2+ ions in modulating changes induced in bean plants by an excess of Cu2+ ions. Chlorophyll fluorescence measurements. Physiol Plant 105:562–568
Mishra D, Kar M (1974) Nickel in plant growth and metabolism. Bot Rev 40:395–452
Mohanty N, Vass J, Demeter S (1989) Impairment of photosystem 2 activity at the level secondary quinone electron acceptor in chloroplasts treated with cobalt, nickel and zinc ions. Physiol Plant 76:389–390
Molas J, (1997) Changes in morphological and anatomical structure of cabbage (Brassica oleracea L.) outer leaves and in ultrastructure of their chloroplast caused by an in vitro excess of nickel. Photosynthetica 34:513–522
Molas J (2002) Changes of chloroplast ultrastructure and total chlorophyll concentration in cabbage leaves caused by excess of organic Ni(II) complexes. Environ Exp Bot 47:115–126
Moustakas M, Ouzounidou G (1994) Increased non-photochemical quenching in leaves of aluminum-stressed wheat plants is due to Al3+-induced elemental loss. Plant Physiol Biochem 32:527–532
Ouzounidou G, Constantinidou HA (1999) Changes in growth and physiology of tobacco and cotton under Ag exposure and recovery: are they of direct or indirect nature? Arch Environ v Contam Toxicol 37:480–487
Ouzounidou G, Moustakas M, Lannoye R (1995) Chlorophyll fluorescence and photoacoustic characteristics in relationship to changes in chlorophyll and Ca2+ content of a Cu-tolerant Silene compacta ecotype under Cu treatment. Physiol Plant 93:551–557
Ouzounidou G, Moustakas M, Eleftheriou EP (1997a) Physiological and ultrastructural effects of cadmium on wheat (Triticum aestivum L.) leaves. Arch Environ Contain Toxicol 32:154–160
Ouzounidou G, Moustakas M, Strasser R (1997b) Sites of action of copper in the photosynthetic apparatus of maize leaves: kinetic analysis of chlorophyll fluorescence, oxygen evolution, absorption changes and thermal dissipation as monitored by photoacoustic signals. Aust J Plant Physiol 24:81–90
Rengel Z (1992) Role of calcium in aluminum toxicity. New Phytol 121:499–513
Robertson AI (1985) The poisoning of roots of Zea mays by nickel ions and protection afforded by magnesium and calcium. New Phytol 100:173–189
Salt DE, Kato N, Krämer U, Smith RD, Raskin I (2000) The role of root exudates in nickel hyperaccumulation and tolerance in accumulator and nonaccumulator species of Thlaspi. In: Terry N, Banuelos G (eds) Phytoremediation of contaminated soil and water. CRC Press LLC, pp 189–200
Sheoran IS, Singal HR, Singh R (1990) Effect of cadmium and nickel on photosynthesis and the enzymes of the photosynthetic carbon reduction cycle in pigeon pea (Cajanus cajan L.). Photosynth Res 23:345–351
Strasser RJ (1978) The grouping model of plant photosynthesis. In: Akoyunoglou G, Argyroudi-Akoyunoglou H (eds) Chloroplast development. Elsevier/North Holland Biomedical Press, Amsterdam, pp 513–542
Tripathy BC, Bhatia B, Mohanty P (1981) Inactivation of chloroplast photosynthetic electron transport activity by Ni2+. Biochim Biophys Acta 638:217–224
Von Caemmerer SV, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and gas exchange of leaves. Planta 153:376–387
Welch RM (1995) Micronutrient nutrition of plants. Crit Rev Plant Sci 14:49–82
Xylander M, Braune W (1994) Influence of nickel on the green-alga Haematococcus-lacustris rostafinski in phases of its life-cycle. J Plant Physiol 114:86–93
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ouzounidou, G., Moustakas, M., Symeonidis, L. et al. Response of Wheat Seedlings to Ni Stress: Effects of Supplemental Calcium. Arch Environ Contam Toxicol 50, 346–352 (2006). https://doi.org/10.1007/s00244-005-5076-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-005-5076-3