Abstract
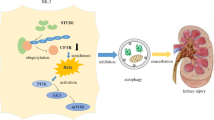
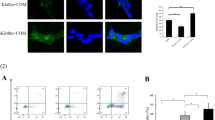
This study was aimed to investigate the preventive effects of N-acetyl-l-cysteine (NAC) against renal tubular cell injury induced by oxalate and stone formation and further explore the related mechanism. Transcriptome sequencing combined with bioinformatics analysis were performed to identify differentially expressed gene (DEG) and related pathways. HK-2 cells were pretreated with or without antioxidant NAC/with or silencing DEG before exposed to sodium oxalate. Then, the cell viability, oxidative biomarkers of superoxidase dismutase (SOD) and malondialdehyde (MDA), apoptosis and cell cycle were measured through CCK8, ELISA and flow cytometry assay, respectively. Male SD rats were separated into control group, hyperoxaluria (HOx) group, NAC intervention group, and TGF-β/SMAD pathway inhibitor group. After treatment, the structure changes and oxidative stress and CaOx crystals deposition were evaluated in renal tissues by H&E staining, immunohistochemical and Pizzolato method. The expression of TGF-β/SMAD pathway related proteins (TGF-β1, SMAD3 and SMAD7) were determined by Western blot in vivo and in vitro. CDKN2B is a DEG screened by transcriptome sequencing combined with bioinformatics analysis, and verified by qRT-PCR. Sodium oxalate induced declined HK-2 cell viability, in parallel with inhibited cellular oxidative stress and apoptosis. The changes induced by oxalate in HK-2 cells were significantly reversed by NAC treatment or the silencing of CDKN2B. The cell structure damage and CaOx crystals deposition were observed in kidney tissues of HOx group. Meanwhile, the expression levels of SOD and 8-OHdG were detected in kidney tissues of HOx group. The changes induced by oxalate in kidney tissues were significantly reversed by NAC treatment. Besides, expression of SMAD7 was significantly down-regulated, while TGF-β1 and SMAD3 were accumulated induced by oxalate in vitro and in vivo. The expression levels of TGF-β/SMAD pathway related proteins induced by oxalate were reversed by NAC. In conclusion, we found that NAC could play an anti-calculus role by mediating CDKN2B/TGF-β/SMAD axis.







Similar content being viewed by others
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
References
Thongprayoon C, Krambeck AE, Rule AD (2020) Determining the true burden of kidney stone disease. Nat Rev Nephrol 16(12):736–746. https://doi.org/10.1038/s41581-020-0320-7
Wang Z, Zhang Y, Zhang J, Deng Q, Liang H (2021) Recent advances on the mechanisms of kidney stone formation (review). Int J Mol Med. https://doi.org/10.3892/ijmm.2021.4982
Narula S, Tandon S, Kumar D, Varshney S, Adlakha K, Sengupta S et al (2020) Human kidney stone matrix proteins alleviate hyperoxaluria induced renal stress by targeting cell-crystal interactions. Life Sci 262:118498. https://doi.org/10.1016/j.lfs.2020.118498
Burns Z, Knight J, Fargue S, Holmes R, Assimos D, Wood K (2020) Future treatments for hyperoxaluria. Curr Opin Urol 30(2):171–176. https://doi.org/10.1097/mou.0000000000000709
Wang J, Chen JJ, Huang JH, Lv BD, Huang XJ, Hu Q et al (2021) Protective effects of total flavonoids from Lysimachia christinae on calcium oxalate-induced oxidative stress in a renal cell line and renal tissue. Evid-Based Complement Alternat Med (eCAM) 2021:6667902. https://doi.org/10.1155/2021/6667902
Sun Y, Dai S, Tao J, Li Y, He Z, Liu Q et al (2020) Taurine suppresses ROS-dependent autophagy via activating Akt/mTOR signaling pathway in calcium oxalate crystals-induced renal tubular epithelial cell injury. Aging 12(17):17353–17366. https://doi.org/10.18632/aging.103730
Luo G, Liu J, Bian T, Zhang Z, Li M (2021) The mechanism of N-acetyl-l-cysteine in improving the secretion of porcine follicle-stimulating hormone in Pichia pastoris. Yeast (Chichester, Engl) 38(11):601–611. https://doi.org/10.1002/yea.3668
Li C, Xie N, Li Y, Liu C, Hou FF, Wang J (2019) N-acetylcysteine ameliorates cisplatin-induced renal senescence and renal interstitial fibrosis through sirtuin1 activation and p53 deacetylation. Free Radical Biol Med 130:512–527. https://doi.org/10.1016/j.freeradbiomed.2018.11.006
Fan H, Le JW, Zhu JH (2020) Protective effect of N-acetylcysteine pretreatment on acute kidney injury in septic rats. J Surg Res 254:125–134. https://doi.org/10.1016/j.jss.2020.04.017
Oksidatif SOR (2020) The efficacy of N-acetylcysteine against renal oxidative stress after extracorporeal shock wave treatment: an experimental rat model. J Urol Surg 7(1):8–15
Desoky EAE, Sakr AM, Alhefnawy M, Omran M, Abdalla MMH, Shahin AS et al (2020) Renal protective effect of N-acetylcysteine with stepwise ramping voltage against extracorporeal shock wave lithotripsy-induced renal injury: a prospective randomized trial. Int Urol Nephrol 52(12):2261–2267. https://doi.org/10.1007/s11255-020-02580-1
Yami A, Hamzeloo-Moghadam M, Darbandi A, Karami A, Mashati P, Takhviji V et al (2020) Ergolide, a potent sesquiterpene lactone induces cell cycle arrest along with ROS-dependent apoptosis and potentiates vincristine cytotoxicity in ALL cell lines. J Ethnopharmacol 253:112504. https://doi.org/10.1016/j.jep.2019.112504
Xu J, Feng ZP, Peng HY, Fu P (2021) Omega-3 polyunsaturated fatty acids alleviate adenine-induced chronic renal failure via regulating ROS production and TGF-β/SMAD pathway. Eur Rev Med Pharmacol Sci 25(22):6825. https://doi.org/10.26355/eurrev_202111_27221
Dou F, Ding Y, Wang C, Duan J, Wang W, Xu H et al (2020) Chrysophanol ameliorates renal interstitial fibrosis by inhibiting the TGF-β/Smad signaling pathway. Biochem Pharmacol 180:114079. https://doi.org/10.1016/j.bcp.2020.114079
Liu WR, Lu HT, Zhao TT, Ding JR, Si YC, Chen W et al (2020) Fu-Fang-Jin-Qian-Cao herbal granules protect against the calcium oxalate-induced renal EMT by inhibiting the TGF-β/smad pathway. Pharm Biol 58(1):1115–1122. https://doi.org/10.1080/13880209.2020.1844241
Li Y, Yu S, Gan X, Zhang Z, Wang Y, Wang Y et al (2017) MRP-1 and BCRP promote the externalization of phosphatidylserine in oxalate-treated renal epithelial cells: implications for calcium oxalate urolithiasis. Urology 107:271.e9-271.e17. https://doi.org/10.1016/j.urology.2017.05.034
Li Y, McMartin KE (2009) Strain differences in urinary factors that promote calcium oxalate crystal formation in the kidneys of ethylene glycol-treated rats. Am J Physiol Renal Physiol 296(5):F1080–F1087. https://doi.org/10.1152/ajprenal.90727.2008
Jewell DE, Tavener SK, Hollar RL, Panickar KS (2022) Metabolomic changes in cats with renal disease and calcium oxalate uroliths. Metabolomics Off J Metabol Soc 18(8):68. https://doi.org/10.1007/s11306-022-01925-4
Albert A, Paul E, Rajakumar S, Saso L (2020) Oxidative stress and endoplasmic stress in calcium oxalate stone disease: the chicken or the egg? Free Radical Res 54(4):244–253. https://doi.org/10.1080/10715762.2020.1751835
Chaiyarit S, Thongboonkerd V (2020) Mitochondrial dysfunction and kidney stone disease. Front Physiol 11:566506. https://doi.org/10.3389/fphys.2020.566506
Carcy R, Cougnon M, Poet M, Durandy M, Sicard A, Counillon L et al (2021) Targeting oxidative stress, a crucial challenge in renal transplantation outcome. Free Radical Biol Med 169:258–270. https://doi.org/10.1016/j.freeradbiomed.2021.04.023
Samadarsi R, Dutta D (2020) Anti-oxidative effect of mangiferin-chitosan nanoparticles on oxidative stress-induced renal cells. Int J Biol Macromol 151:36–46. https://doi.org/10.1016/j.ijbiomac.2020.02.112
Jelic MD, Mandic AD, Maricic SM, Srdjenovic BU (2021) Oxidative stress and its role in cancer. J Cancer Res Ther 17(1):22–28. https://doi.org/10.4103/jcrt.JCRT_862_16
Liang X, Su Y, Huo Y (2021) Forkhead box protein O1 (FoxO1)/SERPINB1 ameliorates ROS production in diabetic nephropathy. Food Sci Nutr 9(1):44–51. https://doi.org/10.1002/fsn3.1859
Peng Z, Chen W, Wang L, Ye Z, Gao S, Sun X et al (2015) Inhalation of hydrogen gas ameliorates glyoxylate-induced calcium oxalate deposition and renal oxidative stress in mice. Int J Clin Exp Pathol 8(3):2680–2689
Khan A, Byer K, Khan SR (2014) Exposure of Madin-Darby canine kidney (MDCK) cells to oxalate and calcium oxalate crystals activates nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase. Urology 83(2):510.e1–7. https://doi.org/10.1016/j.urology.2013.10.038
Chatterjee PK, Cuzzocrea S, Brown PA, Zacharowski K, Stewart KN, Mota-Filipe H et al (2000) Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int 58(2):658–673. https://doi.org/10.1046/j.1523-1755.2000.00212.x
Unno R, Kawabata T, Taguchi K, Sugino T, Hamamoto S, Ando R et al (2020) Deregulated MTOR (mechanistic target of rapamycin kinase) is responsible for autophagy defects exacerbating kidney stone development. Autophagy 16(4):709–723. https://doi.org/10.1080/15548627.2019.1635382
Singh A, Tandon S, Kumar D, Kaur T, Kesari KK, Tandon C (2022) Insights into the cytoprotective potential of Bergenia ligulata against oxalate-induced oxidative stress and epithelial-mesenchymal transition (EMT) via TGFβ1/p38MAPK pathway in human renal epithelial cells. Urolithiasis. https://doi.org/10.1007/s00240-022-01315-4
Chaiyarit S, Thongboonkerd V (2012) Changes in mitochondrial proteome of renal tubular cells induced by calcium oxalate monohydrate crystal adhesion and internalization are related to mitochondrial dysfunction. J Proteome Res 11(6):3269–3280. https://doi.org/10.1021/pr300018c
Yang M, Yin E, Xu Y, Liu Y, Li T, Dong Z et al (2022) CDKN2B antisense RNA 1 expression alleviates idiopathic pulmonary fibrosis by functioning as a competing endogenous RNA through the miR-199a-5p/Sestrin-2 axis. Bioengineered 13(3):7746–7759. https://doi.org/10.1080/21655979.2022.2044252
Lee HA, Chu KB, Moon EK, Quan FS (2021) Histone deacetylase inhibitor-induced CDKN2B and CDKN2D contribute to G2/M Cell cycle arrest incurred by oxidative stress in Hepatocellular carcinoma cells via forkhead box M1 suppression. J Cancer 12(17):5086–5098. https://doi.org/10.7150/jca.60027
Kim SR, Puranik AS, Jiang K, Chen X, Zhu XY, Taylor I et al (2021) Progressive cellular senescence mediates renal dysfunction in ischemic nephropathy. J Am Soc Nephrol 32(8):1987–2004. https://doi.org/10.1681/asn.2020091373
Li Y, Ding T, Hu H, Zhao T, Zhu C, Ding J et al (2021) LncRNA-ATB participates in the regulation of calcium oxalate crystal-induced renal injury by sponging the miR-200 family. Mol Med (Cambridge, MA) 27(1):143. https://doi.org/10.1186/s10020-021-00403-2
Hu H, Chen W, Ding J, Jia M, Yin J, Guo Z (2015) Fasudil prevents calcium oxalate crystal deposit and renal fibrogenesis in glyoxylate-induced nephrolithic mice. Exp Mol Pathol 98(2):277–285. https://doi.org/10.1016/j.yexmp.2015.02.006
Geng XQ, Ma A, He JZ, Wang L, Jia YL, Shao GY et al (2020) Ganoderic acid hinders renal fibrosis via suppressing the TGF-β/Smad and MAPK signaling pathways. Acta Pharmacol Sin 41(5):670–677. https://doi.org/10.1038/s41401-019-0324-7
Acknowledgements
None.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC, No.81370803).
Author information
Authors and Affiliations
Contributions
Conception and design: Ruihua An, Wei Cao, Jingbo Zhang. Data analysis and interpretation: Wei Cao, Shiliang Yu, Xiuguo Gan. Manuscript writing: Wei Cao, Jingbo Zhang, Ruihua An. Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Approval was obtained from the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University and the animal study procedures were conformed to the Animal Care and Use guidelines provided by the National Institutes of Health (NIH).
The experimental protocol was performed in accordance with the relevant guidelines and regulations of the Basel Declaration. The study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, W., Zhang, J., Yu, S. et al. N-acetylcysteine regulates oxalate induced injury of renal tubular epithelial cells through CDKN2B/TGF-β/SMAD axis. Urolithiasis 52, 46 (2024). https://doi.org/10.1007/s00240-023-01527-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00240-023-01527-2