Abstract
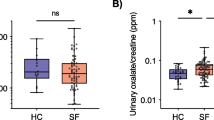
The trillions of microbes that colonize our adult intestine are referred to as the gut microbiome (GMB). Functionally it behaves as a metabolic organ that communicates with, and complements, our own human metabolic apparatus. While the relationship between the GMB and kidney stone disease (KSD) has not been investigated, dysbiosis of the GMB has been associated with diabetes, obesity and cardiovascular disease. In this pilot study we sought to identify unique changes in the GMB of kidney stone patients compared to patients without KSD. With an IRB-approved protocol we enrolled 29 patients into our pilot study. 23 patients were kidney stone formers and six were non-stone forming controls. Specimens were collected after a 6h fast and were flash frozen in dry ice and then stored at −80 °C. Microbiome: determination of bacterial abundance was by analysis of 16 s rRNA marker gene sequences using next generation sequencing. Sequencing of the GMB identified 178 bacterial genera. The five most abundant enterotypes within each group made up to greater than 50 % of the bacterial abundance identified. Bacteroides was 3.4 times more abundant in the KSD group as compared to control (34.9 vs 10.2 %; p = 0.001). Prevotella was 2.8 times more abundant in the control group as compared to the KSD group (34.7 vs 12.3 %; p = 0.005). In a multivariate analysis including age, gender, BMI, and DM, kidney stone disease remained an increased risk for high prevalence for Bacteroides (OR = 3.26, p = 0.033), whereas there was an inverse association with Prevotella (OR = 0.37, p = 0.043). There were no statistically significant differences in bacterial abundance levels for Bacteroides or Prevotella when comparing patients with and without DM, obesity (BMI >30), HTN or HLD. 11 kidney stone patients completed 24 h urine analysis at the time of this writing. Looking at the bacterial genuses with at least 4 % abundance in the kidney stone group, Eubacterium was inversely correlated with oxalate levels (r = −0.60, p < 0.06) and Escherichia trended to an inverse correlation with citrate (r = −0.56, p < 0.08). We also compared bacterial abundance between uric acid (UA) stone formers (n = 5) and non UA stone formers (n = 18) and found no significant difference between them. We identified two genus of bacteria in the GMB that had significant association with KSD. Interestingly, components of the 24-h urine appear to be correlated to bacterial abundance. These preliminary studies for the first time associate differences in the GMB with kidney stone formation. Further studies are warranted to evaluate the potential causative role of preexisting dysbiosis in kidney stone disease.




Similar content being viewed by others
References
Scales CD Jr, Smith AC, Hanley JM, Saigal CS, Urologic diseases in America P (2012) Prevalence of kidney stones in the United States. Eur Urol 62:160–165
Antonelli JA, Maalouf NM, Pearle MS, Lotan Y (2014) Use of the National Health and Nutrition Examination Survey to calculate the impact of obesity and diabetes on cost and prevalence of urolithiasis in 2030. Eur Urol 66:724–729
Lange JN, Mufarrij PW, Wood KD, Holmes RP, Assimos DG (2012) The association of cardiovascular disease and metabolic syndrome with nephrolithiasis. Curr Opin Urol 22:154–159
Rule AD, Roger VL, Melton LJ 3rd et al (2010) Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol 21:1641–1644
Domingos F, Serra A (2011) Nephrolithiasis is associated with an increased prevalence of cardiovascular disease. Nephrol Dial Transplant 26:864–868
Ferraro PM, Taylor EN, Eisner BH et al (2013) History of kidney stones and the risk of coronary heart disease. JAMA J Am Med Assoc 24(310):408–415
Taylor EN, Stampfer MJ, Curhan GC (2004) Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol 15:3225–3232
Tracy CR, Best S, Bagrodia A et al (2014) Animal protein and the risk of kidney stones: a comparative metabolic study of animal protein sources. J Urol 192:137–141
Pak CY (1998) Kidney stones. Lancet 13(351):1797–1801
Borghi L, Schianchi T, Meschi T et al (2002) Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 10(346):77–84
Suez J, Korem T, Zeevi D et al (2014) Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 9(514):181–186
Everard A, Cani PD (2013) Diabetes, obesity and gut microbiota. Best Pract Res Cl Ga. 27:73–83
Andrade-Oliveira V, Amano MT, Correa-Costa M, et al (2015) Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol
Wang Z, Klipfell E, Bennett BJ et al (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 7(472):57–63
Heimann E, Nyman M, Degerman E (2015) Propionic acid and butyric acid inhibit lipolysis and de novo lipogenesis and increase insulin-stimulated glucose uptake in primary rat adipocytes. Adipocyte 4:81–88
Wang Y, Qian PY (2009) Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS One 4:e7401
Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R (2008) Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat Methods 5:235–237
Smith BC, McAndrew T, Chen Z et al (2012) The cervical microbiome over 7 years and a comparison of methodologies for its characterization. PLoS One 7:e40425
Caporaso JG, Kuczynski J, Stombaugh J et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 1(26):2460–2461
Matsen FA, Kodner RB, Armbrust EV (2010) pplacer: linear time maximum-likelihood and Bayesian phylogenetic placement of sequences onto a fixed reference tree. BMC Bioinform 11:538
Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microb 71:8228–8235
Karlsson FH, Tremaroli V, Nookaew I et al (2013) Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 6(498):99–103
Qin J, Li Y, Cai Z et al (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 4(490):55–60
Vrieze A, Van Nood E, Holleman F et al (2012) Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143(913–6):e7
Vitetta L, Linnane AW, Gobe GC (2013) From the gastrointestinal tract (GIT) to the kidneys: live bacterial cultures (probiotics) mediating reductions of uremic toxin levels via free radical signaling. Toxins 5:2042–2057
Khan SR (2013) Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urology 189:803–811
Puddu A, Sanguineti R, Montecucco F, Viviani GL (2014) Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediat Inflamm 2014:162021
De Filippo C, Cavalieri D, Di Paola M et al (2010) Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci 17(107):14691–14696
David LA, Maurice CF, Carmody RN et al (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 23(505):559–563
Wu GD, Chen J, Hoffmann C et al (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science 7(334):105–108
Dethlefsen L, Relman DA (2011) Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci 108(Suppl 1):4554–4561
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by The Montefiore Department of Urology.
Conflict of interest
The authors declare that they have no conflict of interest.
Animals
No animal use for this paper.
Ethical approval
All specimen retrieval involving human participants were in accordance with the ethical standards of the Albert Einstein College of medicine institutional review board with approval number 2014-3487 and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. Consent was approved by the Albert Einstein College of medicine institutional review board with approval number 2014-3487.
Rights and permissions
About this article
Cite this article
Stern, J.M., Moazami, S., Qiu, Y. et al. Evidence for a distinct gut microbiome in kidney stone formers compared to non-stone formers. Urolithiasis 44, 399–407 (2016). https://doi.org/10.1007/s00240-016-0882-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-016-0882-9