Abstract
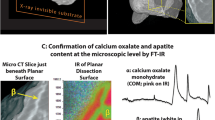
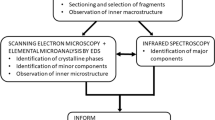
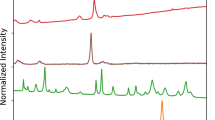
The mechanism(s) by which crystals are retained in the kidney resulting in stone disease remains unclear. Intratubular aggregation as well as crystal cell binding, or internalization and translocation, or alternatively nucleation and growth in the interstitial fluid are possible models. Our group is testing the hypothesis that calcium phosphate deposits in kidneys of patients with calcium renal stones arise in unique anatomical regions of the kidney. Furthermore, we believe that their formation is conditioned by specific stone forming pathophysiologies. To test this hypothesis, we performed intra-operative renal papillary biopsies during percutaneous nephrolithotomy of kidneys from 15 idiopathic calcium stone formers as well as kidney tissue from a patient who ingested ethylene glycol, and developed a new protocol to accurately identify the composition of the calcium deposits located in the renal tissue. We developed a new histological approach that incorporated a low-energy (low-E) reflective slide substrate that has similar characteristics to a common microscope slide and infrared absorption microspectroscopy. Infrared absorption microspectroscopy revealed the crystal deposits in the idiopathic calcium oxalate stone formers to be hydroxyapatite in composition with an occasional region of calcium carbonate, while calcium oxalate was the predominant mineral in the kidney of the patient who had ingested ethylene glycol. The results demonstrate that mixed sample types containing tissue and mineralized deposits are easily analyzed while mounted on a low-E slide using the attenuated total internal reflectance (ATR) method. Reflection/absorption (R/A) analysis allows one to quickly survey a tissue section and provides qualitative information about its components. Once interesting sites have been identified by R/A analysis, ATR analysis can then be used to collect the best data possible. ATR analysis provides spectra free from many of the artifacts associated with transmission and R/A analysis, and completes the full picture of the components contained in the crystal deposits and tissue. We present a method of analysis for mineralized materials embedded in kidney tissue that uses readily or easily obtainable materials and instrumentation. The sensitivity of this method allows tissue sections to remain unstained, alleviating the tedious and time-consuming constraints of earlier methods of visual analysis. The present method will save time and training, while simultaneously offering an unbiased analysis of mineralized components that is more accurate and conducive to patient treatments than previous methods.





Similar content being viewed by others
References
Huleihel M, Erukhimovitch V, Talyshinsky M, Karpasas M (2002) Spectroscopic characterization of normal primary and malignant cells transformed by retroviruses. Appl Spectrosc 56: 640
McIntosh L, Mansfield J, Crowson N, Manstch H, Jackson M (1999) Analysis and interpretation of infrared microscopic maps: visualization and classification of skin components by digital staining and multivariate analysis. Biospectroscopy 5: 265
Haka A, Kidder L, Lewis EN (2001) A combined spectroscopic imaging and chemometric approach for automatically partitioning tissue types in human prostate tissue biopsies. Proceedings of SPIE 4259: 47
Heise HM (2001) Clinical applications of near- and mid-infrared spectroscopy. In: Gremlich H-U, Yan B (eds) Infrared and Raman spectroscopy of biological materials. Marcel Dekker, New York
Kidder L, Haka A, Faustino P, Lester D, Levin I, Lewis EN (1998) Infrared spectroscopic imaging as a tool for pathology. Proceedings of SPIE 3257: 178
Chiriboga L, Yee H, Diem M (2000) Infrared spectroscopy of human cells and tissue. part vi: a comparative study of histopathology and infrared microspectroscopy of normal, cirrhotic and cancerous liver tissue. Appl Spectrosc 54: 1
Dukor R, Liebman M, Johnson B (1998) A new, non-destructive method for the analysis of clinical samples with FT-IR microspectroscopy. Cell Molec Biol 44: 211
Yano K, Ohoshima S, Gotou Y, Kumaido K, Moriguchi L, Katayama H (2000) Direct measurement of human lung cancerous and noncancerous tissues by Fourier transform infrared microscopy: can an infrared microscopy be used as a clinical tool? Anal Biochem 287:218–225
Strobel H, Heineman W (1989) Chemical instrumentation: a systematic approach. Wiley-Interscience, New York
Corns C (1983) Infrared analysis of renal calculi: a comparison with conventional techniques. Ann Clin Biochem 20: 20
Carmona P, Bellanato J, Escolar E (1997) infrared and raman spectroscopy of urinary calculi: a review. Biospectroscopy 3: 331
Volmer M, DeVries J, Goldschmidt H (2001) Infrared analysis of urinary calculi by a single reflection accessory and a neural network interpretation algorithm. Clin Chem 47: 1287
Lehmann C, McClure G, Smolens I (1988) Identification of renal calculi by computerized infrared spectroscopy. Clin Chim Acta 173: 107
Estepa L, Levillain P, Lacour B, Daudon M (1999) Infrared analysis of urinary stones: a trial of automated identification. Clin Chem Lab Med 37: 1043
Diem M, Chiriboga L, Lasch P, Pacifico A (2002) IR spectra and IR spectral maps of individual normal and cancerous cells. Biopolymers (Biospectroscopy) 67: 349
Diem M, Chiriboga L, Pacifico A, Yee H (2000) Infrared microspectroscopy of cells and tissue: infrared spectra and infrared spectral maps of human tissues. Inst Phys Conf Ser 165: 77
Diem M, Chiriboga L, Yee H (2000) Infrared spectroscopy of human cells and tissue. VIII: Strategies for analysis of infrared tissue mapping and applications to liver tissue. Biospectroscopy 57: 282
Aparicio S, Doty B, Camacho N, Paschalis E, Spevak L, Mendelsohn R, Boskey A (2002) Optimal methods for processing mineralized tissues for fourier transform infrared microspectroscopy. Calcif Tissue Int 70: 422
Mendelsohn R, Chen H-C, Rerek M, Moore D (2003) Infrared microspectroscopic imaging maps the spatial distribution of exogenous molecules in skin. J Biomed Optics 8: 185
Marcott C, Reeder R, Paschalis E, Boskey A, Mendelsohn R (1999) Infrared microspectroscopic imaging of biomineralized tissues using a mercury-cadmium-telluride focal-plane array detector. Phosphorus, Sulfur Silicon 144–146: 417
Chiriboga L, Yee H, Diem M (2000) Infrared spectroscopy of human cells and tissues. part vii: ft-ir microspectroscopy of dnase- and rnase-treated normal, cirrhotic, and neoplastic liver tissue. Appl Spectrosc 54: 480
Ouyang H, Sherman P, Paschalis E, Boskey A, Mendelsohn R (2004) Fourier transform infrared microscopic imaging: effects of estrogen and estrogen deficiency on fracture healing in rat femurs. Appl Spectrosc 58: 1
Evan A, Lingeman J, Coe F, Parks J, Bledsoe S, Shao Y, Sommer A, Paterson R, Kuo R, Grynpas M (2003) Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 111: 607
Diem M, Boydston-White S, Chiriboga L (1999) Infrared spectroscopy of cells and tissues: shining light onto a novel subject. Appl Spectrosc 53: 148A
Yasue T (1969) Histochemical identification of calcium oxalate. Acta Histochem Cytochem 2: 83
Cohen J, Virnelson C (1992) FT-IR spectrometer window and method, in USPTO, US, Tremco Incorporated, 1992, US Patent 5,160,826
Bruker Optics Guide for Infrared Spectroscopy. Bruker Optics, Billerica
Bubendorf L, Nocito A, Moch H, Sauter G (2001) Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. J Pathol 195: 72
Kononen J, Hostetter G, Sauter G, Kallioniemi O-P, Brand K, Trivett M (2003) Tissue microarrays. DNA Microarrays 603–607, 644–645
Hedvat C V (2003) Tissue arrays. Expr Profiling Hum Tumors 61–72
Zhang X-P, Su D, Cheng Q-H (2003) Advantages and applications of tissue microarray technology on cancer research. Chin J Cancer Res 15: 74
Skacel M, Skilton B, Pettay JD, Tubbs RR (2002) Tissue microarrays: a powerful tool for high-throughput analysis of clinical specimens: a review of the method with validation data. Appl Immunohistochem Mol Morphol 10:1–6
Rimm DL, Camp RL, Charette LA, Costa J, Olsen DA, Reiss M (2001) Tissue microarray: a new technology for amplification of tissue resources. Cancer J 7: 24
Dukor R, Marcott C (2001) Method and system for performing infrared study on a biological sample, in USPTO, US, VYsis, Inc, US Patent 6,274,871 B1
Patterson B, Danielson N, Sommer A (2003) Attenuated total internal reflectance infrared microspectroscopy as a detection technique for high-performance liquid chromatography. Anal Chem 75: 1418
Dukor RK (1999) Method of identifying cellular types in a biological sample supported on an absorptive substrate by infrared spectroscopy, in USPTO, US, Vysis Inc., 1999, US Patent 5,945,674
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anderson, J., Dellomo, J., Sommer, A. et al. A concerted protocol for the analysis of mineral deposits in biopsied tissue using infrared microanalysis. Urol Res 33, 213–219 (2005). https://doi.org/10.1007/s00240-004-0456-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-004-0456-0