Abstract
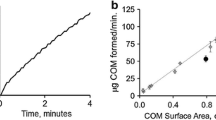
Based on the structure of kidney stones, it is likely that they form as aggregations of preformed crystals, mostly calcium oxalate monohydrate (COM). In this study, we examined the ability of a macromolecular mixture isolated from the urine of normal individuals and stone formers to inhibit aggregation of preformed COM seed crystals in a simple ionic solution using measurements of changes in the particle size distribution (PSD) of preformed COM crystal aggregates. We also examined the effect in this assay of a number of synthetic homopolymers, naturally occurring urine macromolecules, and binary mixtures thereof. The macromolecular mixtures from urine of normals and most stone formers reduced the degree of aggregation of the seed crystals, whereas 22% of stone former urine macromolecules either did not disaggregate or actually promoted further aggregation. Stone formers within one family shared this property, but a non-stone forming sibling did not. Polyanions, either synthetic or naturally occurring, induced disaggregation to an extent similar to that exhibited by normal urine macromolecules, while polycations had no effect on the PSD. However, mixing a polyanion, either poly-aspartate or osteopontin, with the polycation poly-arginine, changed their behavior from disaggregation to aggregation promotion. The disaggregating behavior of normal urinary macromolecules provides a defense against aggregation, but a minority of stone forming individuals lacks this defense, which may contribute to stone formation.




Similar content being viewed by others
References
Asplin JR, Parks JH, Chen MS, Lieske JC, Toback FG, Pillay SN, Nakagawa Y, Coe FL (1999) Reduced crystallization inhibition by urine from men with nephrolithiasis. Kidney Int 56:1505
Atmani F, Mizon J, Khan SR (1996) Identification of uronic-acid-rich protein as urinary bikunin, the light chain of inter-alpha-inhibitor. Eur J Biochem 236:984
Beshensky AM, Wesson JA, Worcester EM, Sorokina EJ, Snyder CJ, Kleinman JG (2001) Effects of urinary macromolecules on hydroxyapatite crystal formation. J Am Soc Nephrol 12:2108
Dorian HH, Rez P, Drach GW (1996) Evidence for aggregation in oxalate stone formation: atomic force and low voltage scanning electron microscopy. J Urol 156:1833
Ebisuno S, Kohjimoto Y, Yoshida T, Ohkawa T (1993) Effect of urinary macromolecules on aggregation of calcium oxalate in recurrent calcium stone formers and healthy. Urol Res 21:265
Grases F, Costa-Bauza A, Conte A (1993) Studies on structure of calcium oxalate monohydrate renal papillary calculi. Mechanism of formation. Scanning Microsc 7:1067
Guo S, Ward MD, Wesson JA (2002) Direct visualization of calcium oxalate monohydrate crystallization and dissolution with atomic force microscopy and the role of polymeric additives. Langmuir 18:4284
Khan SR, Hackett RL (1993) Role of organic matrix in urinary stone formation: an ultrastructural study of crystal matrix interface of calcium oxalate monohydrate stones. J Urol 150:239
Kok DJ, Papapoulos SE, Bijvoet OL (1990) Crystal agglomeration is a major element in calcium oxalate urinary stone formation. Kidney Int 37:51
Nakagawa Y, Abram V, Kezdy FJ, Kaiser ET, Coe FL (1983) Purification and characterization of the principal inhibitor of calcium oxalate monohydrate crystal growth in human urine. J Biol Chem 258:12594
Nancollas GH, Smesko SA, Campbell AA, Richardson CF, Johnsson M, Iadiccico RA, Binette JP, Binette M (1991) Physical chemical studies of calcium oxalate crystallization. Am J Kidney Dis 17:392
Pak CY, Holt K (1976) Nucleation and growth of brushite and calcium oxalate in urine of stone-formers. Metabolism 25:665
Robertson WG, Peacock M (1972) Calcium oxalate crystalluria and inhibitors of crystallization in recurrent renal stone-formers. Clin Sci 43:499
Robertson WG, Peacock M, Marshall RW, Marshall DH, Nordin BE (1976) Saturation-inhibition index as a measure of the risk of calcium oxalate stone formation in the urinary tract. N Engl J Med 294:249
Robertson WG, Peacock M, Nordin BEC (1969) Calcium crystalluria in recurrent renal stone formers. Lancet 21
Ryall RL, Grover PK, Stapleton AM, Barrell DK, Tang Y, Simpson RJ (1995) The urinary F1 activation peptide of human prothrombin is a potent inhibitor of calcium oxalate crystallization in undiluted human urine in vitro. Clin Sci 89:533
Ryall RL, Harnett RM, Marshall VR (1981) The effect of urine, pyrophosphate, citrate, magnesium and glycosaminoglycans on the growth and aggregation of calcium oxalate crystals in vitro. Clin Chim Acta 112:349
Sheng X, Ward MD, Wesson JA (2003) Adhesion between molecules and calcium oxalate crystals: critical interactions in kidney stone formation. J Am Chem Soc 125:2854
Shiraga H, Min W, VanDusen WJ, Clayman MD, Miner D, Terrell CH, Sherbotie JR, Foreman JW, Przysiecki C, Neilson EG, Hoyer JR (1992) Inhibition of calcium oxalate crystal growth in vitro by uropontin: another member of the aspartic acid-rich protein superfamily. Proc Natl Acad Sci U S A 89:426
Sorensen ES, Petersen TE (1993) Purification and characterization of three proteins isolated from the proteose peptone fraction of bovine milk. J Dairy Res 60:189
Springmann KE, Drach GW, Gottung B, Randolph AD (1986) Effects of human urine on aggregation of calcium oxalate crystals. J Urol 135:69
Thongboonkerd V, McLeish KR, Arthur JM, Klein JB (2002) Proteomic analysis of normal human urinary proteins isolated by acetone precipitation or ultracentrifugation. Kidney Int 62:1461
Wesson JA, Worcester EM, Wiessner JH, Mandel NS, Kleinman JG (1998) Control of calcium oxalate crystal structure and cell adherence by urinary macromolecules. Kidney Int 53:952
Worcester EM, Blumenthal SS, Beshensky AM, Lewand DL (1992) The calcium oxalate crystal growth inhibitor protein produced by mouse kidney cortical cells in culture is osteopontin. J Bone Miner.Res 7:1029
Acknowledgments
Supported by new investigator funds from Medical College of Wisconsin (J.A.W), a Career Development Award from the Department of Veterans Affairs (J.A.W.), and the N.I.H. (DK-48504; J.G.K.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wesson, J.A., Ganne, V., Beshensky, A.M. et al. Regulation by macromolecules of calcium oxalate crystal aggregation in stone formers. Urol Res 33, 206–212 (2005). https://doi.org/10.1007/s00240-004-0455-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-004-0455-1