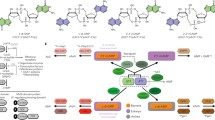
Abstract
All known alarmones are ribonucleotides or ribonucleotide derivatives that are synthesized when cells are under stress conditions, triggering a stringent response that affects major processes such as replication, gene expression, and metabolism. The ample phylogenetic distribution of alarmones (e.g., cAMP, Ap(n)A, cGMP, AICAR, and ZTP) suggests that they are very ancient molecules that may have already been present in cellular systems prior to the evolutionary divergence of the Archaea, Bacteria, and Eukarya domains. Their chemical structure, wide biological distribution, and functional role in highly conserved cellular processes support the possibility that these modified nucleotides are molecular fossils of an epoch in the evolution of chemical signaling and metabolite sensing during which RNA molecules played a much more conspicuous role in biological catalysis and genetic information.






Similar content being viewed by others
Notes
The PyMol Molecular Graphics System, Version 2.0 Schrödinger, LLC.
References
Abranches J, Martinez AR, Kajfasz JK, Chávez V, Garsin DA, Lemos JA (2009) The molecular alarmone (p)ppGpp mediates stress responses, vancomycin tolerance, and virulence in Enterococcus faecalis. J Bacteriol 191:2248–2256
Artymiuk PJ, Poirrette AR, Rice DW, Willett P (1997) A polymerase I palm in adenylyl cyclase? Nature 388:33–34
Atkinson GC, Tenson T, Hauryliuk V (2011) The RelA/SpoT homolog (RSH) superfamily: distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PloS ONE 6:e23479
Bailey S, Wing RA, Steitz TA (2006) The structure of T. aquaticus DNA polymerase III is distinct from eukaryotic replicative DNA polymerase. Cell 126:893–904
Baker DA, Kelly JM (2004) Structure, function and evolution of microbial adenylyl and guanylyl cyclases. Mol Microbiol 52:1229–1242
Balodimos IA, Kashket ER, Rapaport E (1988) Metabolism of adenylylated nucleotides in Clostridium acetobutylicum. J Bacteriol 170:2301–2305
Bassler J, Schultz JE, Lupas AN (2018) Adenylate cyclases: receivers, transducers, and generators of signals. Cell Signal 46:135–144
Battesti A, Bouveret E (2006) Acyl carrier protein/SpoT interaction, the switch linking SpoT-dependent stress response to fatty acid metabolism. Mol Microbiol 62:1048–1063
Bazurto JV, Heitman NJ, Downs DM (2015) Aminoimidazole carboxamide ribotide exerts opposing effects on thiamine synthesis in Salmonella enterica. J Bacteriol 197:2821–2830
Becerra A, Delaye L, Islas S, Lazcano A (2007) The very early stages of biological evolution and the nature of the last common ancestor of the three major cell domains. Annu Rev Ecol Evol Syst 38:361–379
Berleman JE, Hasselbring BM, Bauer CE (2004) Hypercyst mutants in Rhodospirillum centenum identity regulatory loci involved in cyst cell differentiation. J Bacteriol 186:5834–5841
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shinyalov IN, Bourne PE (2000) The protein data bank. Nucleic Acids Res 28:235–242
Bharati BK, Chatterji D (2013) Quorum sensing and pathogenesis: role of small signaling molecules in bacterial persistence. Curr Sci 105:643–656
Bierger B, Essen LO (2001) Structural analysis of adenylate cyclases from Trypanosoma brucei in their monomeric state. EMBO J 20:433–445
Bochner BR, Ames BN (1982) ZTP (5-amino 4-imidazole carboxamide riboside 5′-triphosphate): a proposed alarmone for 10-formyl-tetrahydrofolate deficiency. Cell 29:929–937
Bochner BR, Lee PC, Wilson SW, Cutler CW, Ames BN (1984) AppppA and related adenylylated nucleotides are synthesized as a consequence of oxidation stress. Cell 37:225–232
Bonaventura C, Cashon R, Colacino JM, Hilderman RH (1992) Alteration of hemoglobin function by diadenosine 5′,5″-PP1,P4-tetraphosphate and other alarmones. J Biol Chem 267:4652–4657
Boratyn GM, Schäffer AA, Agarwala R, Altschul SF, Lipman DJ, Madden TL (2012) Domain enhanced lookup time accelerated BLAST. Biol Direct 7:1–14
Breaker RR (2010) RNA second messengers and riboswitches: relics from the RNA World? Microbe 5:13–20
Breaker RR (2012) Riboswitches and the RNA world. Cold Spring Harb Perspect Biol 4:a003566
Callahan MP, Smith KE, Cleaves IIHJ, Ruzicka J, Stern JC, Glavin DP, House CH, Dworkin JP (2011) Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc Natl Acad Sci USA 108:13995–13998
Cashel M, Gallant J (1974) Cellular regulation of guanosine tetraphosphate and guanosine pentaphosphate. In: Nomura M, Tissières A, Lengyel P (eds) Ribosomes. Cold Spring Harbor, New York, pp 733–745
Chatterji D, Ojha AK (2001) Revisiting the stringent response, ppGpp and starvation signaling. Curr Opin Microbiol 4:160–165
Chen J, Gottesman S (2014) Riboswitch regulates RNA. Science 345:876–877
Chen Z-H, Schaap P (2012) The prokaryote messenger c-di-GMP triggers stalk cell differentiation in Dictiostelium. Nature 488:680–683
Chen X, Li N, Ellington AD (2007) Ribozyme catalysis of metabolism in the RNA world. Chem Biodivers 4:633–655
Copper G, Kimmich N, Belisle W, Sarinana J, Brabham K, Garrel L (2001) Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature 414:879–883
Corrigan RM, Gründling A (2013) Cyclic di-AMP: another second messenger enters the fray. Nat Rev Microbiol 11:513–524
Corrigan RM, Campeotto I, Jeganathan T, Roelofs KG, Lee VT, Gründling A (2013) Systematic identification of conserved bacterial c-di-AMP receptor proteins. Proc Natl Acad Sci USA 110:9084–9089
D’Ari R, Casadesús J (1998) Underground metabolism. Bioessays 20:181–186
Daignan-Fornier B, Pinson B (2012) 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranosyl 5′-monophosphate (AICAR), a highly conserved purine intermediate with multiple effects. Metabolites 2:292–302
Dalebroux ZD, Svensson SL, Gaynor EC, Swanson MS (2010) ppGpp conjures bacterial virulence. Microbiol Mol Biol Rev 74:171–199
Davies BW, Bogard RW, Young TS, Mekalanos JJ (2012) Coordinated regulation of accessory genetic elements produces cyclic di-nucleotides for V. cholerae virulence. Cell 149:358–370
De la Fuente-Nuñez C, Reffuveille F, Haney EF, Straus SK, Hancock RE (2014) Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog 10:e1004152
Eakin RE (1963) An approach to the evolution of metabolism. Proc Natl Acad Sci USA 49:360–366
Felsenstein J (2005) PHYLIP (phylogeny inference package) version 3.6. Distributed by the author. Department of Genome Sciences, University of Washington, Seattle
Ferris JP, Joshi PC, Edelson EH, Lawless JG (1978) HCN: a plausible source of purines, pyrimidines and amino acids on the primitive earth. J Mol Evol 11:293–311
Flärdh K, Axberg T, Albertson NH, Kjelleberg S (1994) Stringent control during carbon starvation of marine Vibrio sp. strain S14: molecular cloning, nucleotide sequence, and deletion of the relA gene. J Bacteriol 176:5949–5957
Flores NA, Stavrou BM, Sheridan DJ (1999) The effects of diadenosine polyphosphate on the cardiovascular system. Cardiovasc Res 42:15–26
Gallant J, Palmer L, Pao CC (1977) Anomalous synthesis of ppGpp in growing cells. Cell 11:181–185
Garrison PN, Mathis SA, Barnes LD (1986) In vivo levels of diademosine tetraphosphate and adenosine tetraphospho-guanosine in Physarum polycephalum during the cell cycle and oxidative stress. Mol Cell Biol 6:1179–1186
Geiger T, Wolz C (2014) Intersection of the stringent response and the CodY regulon in low GC gram-positive bacteria. Int J Med Microbiol 304:150–155
Geiger T, Francois P, Liebeke M, Fraunholz M, Goerke C, Krismer B, Schrenzel J, Lalk M, Wolz C (2012) The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular PSMs expression. PLoS Pathog 8:e1003016
Gerdes K, Maisonneuve E (2015) Remarkable functional convergence: alarmone ppGpp mediates persistence by activating type I and II toxin–antitoxins. Mol Cell 59:1–3
Gilbert W (1986) Origin of life: the RNA world. Nature 319:618
Gomelsky M (2011) cAMP, c-di-GMP, c-di-AMP and now cGMP: bacteria use them all! Mol Microbiol 79:562–565
González-Plaza JJ (2018) Small RNAs in cell-to-cell- communications during bacterial infection. FEMS Microbiol Lett 365:1–9
Hall J, Ralph EC, Shanker S, Wang H, Byrnes LJ, Horst R, Wong J, Brault A, Dumlao D, Smith JF, Dakin LA, Schmitt DC, Trujillo J, Vincent F, Griffor M, Aulabaugh AE (2017) The catalytic mechanism of cyclic GMP-AMP synthase (cGAS) and implications for innate immunity and inhibition. Protein Sci 26:2367–2380
Handler P (1961) Evolution of the coenzymes. In: Oparin AI (ed) Proceedings of the 5th International Congress of Biochemistry. Macmillan, New York, pp 149–157
Haseltine WA, Block R (1973) Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosome. Proc Natl Acad Sci USA 70:1564–1568
Hauryliuk V, Atkinson GC, Murakami KS, Tenson T, Gerdes K (2015) Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat Rev Microbiol 13:298–309
Hood EE, Armour S, Ownby JD, Handa AK, Bressan RA (1979) Effect of nitrogen starvation on the level of adenosine 3′,5′-monophosphate in Anabaena variabilis. Biochim Biophys Acta 588:193–200
Huang F, Bugg CW, Yarus M (2000) RNA-catalyzed CoA, NAD, and FAD synthesis from phosphopantetheine, NMN, and FMN. Biochemistry 39:15548–15555
Jácome R, Becerra A, de León SP, Lazcano A (2015) Structural analysis of monomeric RNA-dependent polymerases: evolutionary and therapeutic implications. PLoS ONE 10:e0139001
Jenal U, Reinders A, Lori C (2017) Cyclic di-GMP: second messenger extraordinaire. Nat Rev Microbiol 15:271–284
Jones CP, Ferré-D’Amaré AR (2015) Recognition of the bacterial alarmone ZMP through long-distance association of two RNA subdomains. Nat Struct Mol Biol 22:679–685
Kanehisa M, Goto S (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30
Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M (2016) KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res 44:457–462
Kasahara M, Ohmori M (1999) Activation of a cyanobacterial adenylate cyclase, CyaC, by autophosphorylation and subsequent phosphotransfer reaction. J Biol Chem 274:15167–15172
Kellenberger CA, Wilson SC, Hickey SF, Gonzalez TL, Su Y, Hallberg ZF, Brewer TF, Lavarone AT, Carlson HK, Hsieh YF, Hammond MC (2015) GEMM-I riboswitches from geobacter sense the bacterial second messenger cyclic AMP-GMP. Proc Natl Acad Sci USA 112:5383–5388
Krissinel E, Henrick K (2004) Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr D Biol Crystallogr 60:2256–2268
Krol E, Becker A (2011) ppGpp in Sinorhizobium meliloti: biosynthesis in response to sudden nutritional downshifts and modulation of transcriptome. Mol Microbiol 81:1233–1254
Lamers MH, Georgescu RE, Lee SG, O´Donnell M, Kuriyan J (2006) Crystal structure of the catalytic α subunit of E. coli replicative DNA polymerase III. Cell 126:881–892
Lazcano A (2014) The RNA World: stepping out of the shadows. In: Gabriel T (ed) Why does evolution matter? The importance of understanding evolution. Cambridge Scholars Publishing, Newcastle upon Tyne, pp 101–119
Lazcano A (2018) Prebiotic evolution and self assembly of nucleic acids. ACS Nano. https://doi.org/10.1021/acsnano.8b07605
Lazcano A, Becerra A, Delaye L (2011) Alarmones. In: Margulis L, Asikainen CA, Krumbie WE (ed) Chimeras and consciousness: evolution of the sensory self. The MIT Press, Massachusetts Institute of Technology, Cambridge, pp 35–43
Lee PC, Bochner BR, Ames BN (1983a) Diadenosine 5′,5‴-P1,P4-tetraphosphate and related adenylyllated nucleotides in Salmonella typhimurium. J Biol Chem 258:6827–6834
Lee PC, Bochner BR, Ames BN (1983b) AppppA, heat-shock stress, and cell oxidation. Proc Natl Acad Sci USA 80:7496–7500
Letunic I, Bork P (2016) Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44:242–245
Lori C, Ozaki S, Steiner S, Böhm R, Abel S, Dubey BN, Schirmer T, Hiller S, Jenal U (2015) Cyclic di-GMP acts as a cell cycle oscillator to drive chromosome replication. Nature 523:236–239
Magnusson LU, Farewell A, Nyström T (2005) ppGpp: a global regulator in Escherichia coli. Trends Microbiol 13:236–242
Maisonneuve E, Gerdes K (2014) Molecular mechanisms underlying bacterial persisters. Cell 157:539–548
Majerfeld I, Puthenvedu D, Yarus M (2016) Cross-backbone templating; ribonucleotides made on poly(C). RNA 22:397–407
McDonough KA, Rodriguez A (2011) The myriad roles of cyclic AMP in microbial pathogens: from signal to sword. Nat Rev Microbiol 10:27–38
Mehne FM, Gunka K, Eilers H, Herzberg C, Kaever V, Stülke J (2013) Cyclic di-AMP homeostasis in Bacillus subtilis: both lack in high level accumulation of the nucleotide are detrimental for cell growth. J Biol Chem 288:2004–2017
Münzel T, Feil R, Mülsch A, Lohmann SM, Hofmann F, Walter U (2003) Physiology and pathophysiology of vascular signaling controlled by cyclic guanosine 3′,5′-cyclic monophosphate-dependent protein kinase. Circulation 108:2172–2183
Nelson JW, Breaker RR (2017) The lost language of the RNA World. Sci Signal 10:1–10
Nelson JW, Sudarsan N, Furukawa K, Weinberg Z, Wang JX, Breaker RR (2013) Riboswitches in eubacteria sense the second messenger c-di-AMP. Nat Chem Biol 9:834–839
Orgel LE (1968) Evolution of the genetic apparatus. J Mol Biol 38:381–393
Orgel LE, Sulston JE (1971) Polynucleotide replication and the origin of life. In: Kimball AP, Oro J (ed) Prebiotic and biochemical evolution. North-Holland, Amsterdam, pp 89–94
Oró J (1960) Synthesis of adenine from ammonium cyanide. Biochem Biophys Res Commun 2:407–412
Pendergast W, Yerxa BR, Douglass JG III, Shaver SR, Dougherty RW, Redick CC, Sims IF, Rideout JL (2001) Synthesis and P2Y receptor activity of a series of uridine dinucleoside 5′-polyphosphates. Bioorg Med Chem Lett 11:157–160
Pesavento C, Hengge R (2009) Bacterial nucleotide-based second messengers. Curr Opin Microbiol 12:170–176
Pilz RB, Casteel DE (2003) Regulation of gene expression by cyclic GMP. Circ Res 93:1034–1046
Poole K (2012) Bacterial stress responses as determinants of antimicrobial resistance. J Antimicrob Chemother 67:2069–2089
Potrykus K, Cashel M (2008) (p)ppGpp: still magical? Annu Rev Microbiol 62:35–51
Pullman B (1972) Electronic factors in biochemical evolution. In: Ponnamperuna C (ed) Exobiology. North Holland Publishing Company, Amsterdam, pp 136–169
Puthenvedu D, Janas T, Majerfeld I, Illangasekare M, Yarus M (2015) Poly(u) RNA-templated synthesis of AppA. RNA 21:1818–1825
Rapaport E, Zamecnik PC (1976) Presence of diadenosine 5′,5″-P1,P4-tetraphosphate (Ap4A) in mammals cells in levels varying widely with proliferative activity of the tissue: a possible positive “pleiotypic activator”. Proc Natl Acad Sci USA 73:3984–3988
Rios AC, Tor Y (2013) On the origin of the canonical nucleobases: an assessment of selection pressures across chemical and early biological evolution. Isr J Chem 53:469–483
Ritson D, Sutherland JD (2012) Prebiotic synthesis of simple sugars by photoredox systems chemistry. Nat Chem 4:895–899
Romero D, Traxler MF, López D, Kolter R (2011) Antibiotics as signal molecules. Chem Rev 111:5492–5505
Ross P, Weinhouse H, Aloni Y, Michaeli D, Weinberger-Ohana P, Mayer R, Braun S, de Vroom E, van der Marel GA, van Boom JH, Benziman M (1987) Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 325:279–281
Ryu MH, Moskvin OV, Siltberg-Liberles J, Gomelsky M (2010) Natural and engineered photoactivated nucleotidyl cyclases for optogenetic applications. J Biol Chem 285:41501–41508
Sabina RL, Holmes EW, Becker MA (1984) The enzymatic synthesis of 5-amino-4-imidazolecarboxamide riboside triphosphate (ZTP). Science 16:1193–1195
Saha S, Jia Z, Liu D, Misra HP (2011) The roles of cAMP and G protein signaling in oxidative stress-induced cardiovascular dysfunction. In: Basu S, Wiklund L (ed) Studies on experimental models. Humana Press, Totowa, pp 621–635
Sakaguchi K, Tsujino M, Hayashi M, Kawai K, Mizuno K, Hayano K (1976) Mode of action of bredinin with guanylic acid on L5178Y mouse leukemia cells. J Antibiot 12:1320–1327
Sanders KM, Ward SM (1992) Nitric oxide as a mediator of nonadrenergic, noncholinergic neurotransmission. Am J Physiol 262:379–392
Seifert R, Beste K, Burhenne H, Voigt U, Wolter S, Hammerschmidt A, Reinecke D, Sandner P, Pich A, Schwede F, Genieser HG, Kaever V (2011) Cyclic CMP and cyclic UMP: new (old) second messengers. BMC Pharmacol 11:O34
Shenoy AR, Visweswariah SS (2004) ClassIII nucleotide cyclases in bacteria and archaebacterial: lineage-specific expansion of adenylyl cyclases and a dearth of guanylyl cyclases. FEBS Lett 561:11–21
Shenoy AR, Sivakumar K, Krupa A, Srinivasan N, Visweswariah SS (2004) A survey of nucleotide cyclases in actinobacteria: unique domain organization and expansion of the class III cyclase family in Mycobacterium tuberculosis. Comp Funct Genomics 5:17–38
Sherlock ME, Sudarsan N, Breaker RR (2018) Riboswitches for the alarmone ppGpp expands the collection of RNA-based signaling systems. Proc Natl Acad Sci USA 21:1–6
Sidi Y, Mitchel BS (1985) Z-nucleotide accumulation in erythrocytes from Lesch–Nyhan patients. J Clin Invest 76:2416–2419
Spira B, Silberstein N, Yagil E (1995) Guanosine 3′,5′-bispyrophosphate (ppGpp) synthesis in cells of Escherichia coli starved for Pi. J Bacteriol 177:4053–4058
Stent GS, Brenner S (1961) A genetic locus for the regulation of ribonucleic acid synthesis. Proc Nat Acad Sci USA 47:2005–2014
Stephens JC, Artz SW, Ames BN (1975) Guanosine 5′-diphosphate 3′-diphosphate (ppGpp): positive effector for histidine operon transcription and general signal for amino-acid deficiency. Proc Nat Acad Sci USA 72:4389–4393
Subbiah S, Laurents DV, Levitt M (1993) Structural similarity of DNA-binding domains of bacteriophage repressors and the globin score. Curr Biol 3:141–148
Sudarsan N, Lee ER, Weinberg Z, Moy RH, Kim JN, Link KH, Breaker RR (2008) Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science 18:411–413
Suhadolnik RJ (1979) Naturally occurring nucleoside and nucleotide antibiotics. Prog Nucleic Acid Res Mol Biol 22:193–291
Sun L, Wu J, Du F, Chen F, Chen ZJ (2013) Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339:786–791
Sunahara RK, Beuve A, Tesmer JJ, Sprang SR, Garbers DL, Gilman AG (1998) Exchange of substrate and inhibitor specificities between adenylyl and guanylyl cyclases. J Biol Chem 273:16332–16338
Sureka K, Choi PH, Precit M, Delince M, Pensinger DA, Huynh TN, Jurado AR, Goo YA, Sadilek M, Lavarone AT, Sauer JD, Tong L, Woodward JJ (2014) The cyclic dinucleotide c-di-AMP is an allosteric regulator of metabolic enzyme function. Cell 158:1389–1401
Tamayo R, Pratt JT, Camilli A (2007) Role of cyclic diguanylate in the regulation of bacterial pathogenesis. Annu Rev Microbiol 61:131–148
Thomas CB, Meade JC, Holmes EW (1981) Aminoimidazole carboxamide ribonucleoside toxicity: a model for study of pyrimidine starvation. J Cell Physiol 107:335–344
Vázquez-Salazar A, Lazcano A (2018) Early life: embracing the RNA World. Curr Biol 5:220–222
Vázquez-Salazar A, Tan G, Stockton A, Fani R, Becerra A, Lazcano A (2017) Can an imidazole be formed from an alanyl-seryl-glycine tripeptide under possible prebiotic conditions? Orig Life Evol Biosph 47:345–354
Vázquez-Salazar A, Becerra A, Lazcano A (2018) Evolutionary convergence in the biosyntheses of the imidazole moieties of histidine and purines. PLoS ONE 13:e0196349
Vinella D, Albrecht C, Cashel M, D’Ari R (2005) Iron limitation induces SpoT-dependent accumulation of ppGpp in Escherichia coli. Mol Microbiol 56:958–970
Warner TD, Mitchell JA, Sheng H, Murad F (1994) Effects of cyclic GMP on smooth muscle relaxation. Adv Pharmacol 26:171–194
White HB (1976) Coenzymes as fossils of an earlier metabolic state. J Mol Evol 7:101–104
White HB (1982) Evolution of coenzymes and the origin of pyridine nucleotides. In: Everse J, Anderson B, You KS (ed) The pyridine nucleotide coenzymes. Academic Press, Cambridge, pp. 1–17
Winkler WC, Nahvi A, Roth A, Collins JA, Breaker RR (2004) Control of gene expression by a natural metabolite-responsive ribozyme. Nature 428:281–286
Witte G, Hartung S, Büttner K, Hopfner KP (2008) Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol Cell 30:167–178
Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ (2013) Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339:826–830
Yim G, Wang HH, Davies J (2007) Antibiotics as signaling molecules. Philos Trans R Soc Lond B Biol Sci 362:1195–1200
Zhang L, Li W, He ZG (2013) DarR, a TetR-like transcriptional factor, is a cyclic di-AMP-responsive repressor in Mycobacterium smegmatis. J Biol Chem 288:3085–3096
Acknowledgements
Ricardo Hernández Morales is a doctoral student from the Programa de Doctorado en Ciencias Biológicas, Universidad Nacional Autónoma de México (UNAM). Financial support from PAPIIT-UNAM (IN223916) is gratefully acknowledged. We are indebted to Samuel Ponce de León for many insightful discussions, Alberto Vázquez-Salazar for providing useful references for this work, and José Alberto Campillo Balderas, Rodrigo Jácome, and Adriana Benítez for technical support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Monophyletic origin of the catalytic core of polymerases and adenylate-, guanylate- and diguanylate cyclases. The universal distribution of the palm domain in all living beings suggests this is a very ancient domain which was duplicated and recruited many times during the evolutionary history of polymerases and cyclases. (TIFF 293 KB)
Supplementary Fig. 2
Common origin of the non-canonical palm and the catalytic site of cGAMP synthase. (A) Structural alignment of non-canonical palm domain (1BPY - yellow color) and catalytic site of cGAMP synthase (4TXY – green color). (B) Representation of the monophyletic origin of the catalytic core of polymerase III and β, and cGAMP synthase. (TIFF 547 KB)
Supplementary Table S1
Diversity of alarmones and the biological processes they regulate. Columns include information about the alarmones, the enzymes involved in their biosynthesis and degradation, the stress condition which lead to an increase in their concentration, and the processes that they regulate as described by the reference in the last column. (XLSX 16 KB)
Supplementary Table S2
Distribution (presence-absence) of biosynthetic and degradative enzymes of alarmones in completely sequenced cellular genomes. In first column are enzymes analyzed in this work. Other columns contain the acronyms for each organism’s genome analyzed. Letter “H” indicates that a homologous hit was found in the genome. Letters in each column of the table represent the acronym for each organism’s genome from KEGG database. (XLSX 1654 KB)
Supplementary Table S3
Alarmone biosynthetic and degradative enzymes and their homologous sequences encoded by dsDNA virus with no RNA stage in their biological cycle. Columns, from left to right, indicate: enzyme code (EC), protein identification code, virus type, viral family, and some parameters used on the search. (XLSX 18 KB)
Supplementary Table S4
Protein domains associated with adenylyl cyclases (class III) and their functions. (XLSX 15 KB)
Supplementary Table S5
Polymerase-mediated reaction and the biosynthetic reactions that produce alarmones. (XLSX 11 KB)
Supplementary Table S6
Hypothetical ribozyme-mediated alarmone-biosynthetic reactions. Ribozymes have the ability to catalyze a considerable number of chemical reactions (Table 1). Some of these reactions such as ribozymic polymerization or self-cleaving could lead to the synthesis and accumulation of alarmones. (XLSX 11 KB)
Rights and permissions
About this article
Cite this article
Hernández-Morales, R., Becerra, A. & Lazcano, A. Alarmones as Vestiges of a Bygone RNA World. J Mol Evol 87, 37–51 (2019). https://doi.org/10.1007/s00239-018-9883-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-018-9883-3