Abstract
This paper addresses some questions related to the evolution of cooperative behaviors, in the context of energetic metabolism. Glycolysis can perform either under a dissipative working regime suitable for rapid proliferation or under an efficient regime that entails a good modus operandi under conditions of glucose shortage. A cellular mechanism allowing switching between these two regimes may represent an evolutionary achievement. Thus, we have explored the conditions that might have favored the emergence of such an accommodative mechanism. Because of an inevitable conflict for resources between individual interests and the common good, rapid and inefficient use of glucose is always favored by natural selection in spatially homogeneous environment, regardless of the external conditions. In contrast, when the space is structured, the behavior of the system is determined by its free energy content. If the fuel is abundant, the dissipative strategy dominates the space. However, under famine conditions the efficient regime represents an evolutionary stable strategy in a Harmony game. Between these two extreme situations, both metabolic regimes are engaged in a Prisoner’s Dilemma game, where the output depends on the extracellular free energy. The energy transition values that lead from one domain to another have been calculated. We conclude that an accommodative mechanism permitting alternation between dissipative and efficient regimes might have evolved in heterogeneous and highly fluctuating environments. Overall, the current work shows how evolutionary optimization and game-theoretical approaches can be complementary in providing useful insights into biochemical systems.



Similar content being viewed by others
References
Aledo JC (2004) Glutamine breakdown in rapidly dividing cells: Waste or investment? BioEssays 26:778–785
Aledo JC, Esteban del Valle A (2002) Glycolysis in wonderland: the importance of energy dissipation in metabolic pathways. J Chem Educ 79:1336–1339
Aledo JC, Esteban del Valle A (2004) The ATP paradox is the expression of an economizing fuel mechanism. J Biol Chem 279:55372–55375
Angulo-Brown F, Santillán M, Calleja-Quevedo E (1995) Thermodynamic optimality in some biochemical reactions. Nuevo Cim 17D:87–90
Çakar ZP, Seker UOS, Tamerler C, Sonderegger M, Sauer U (2005) Evolutionary engineering of multiple-stress resistant Saccharomyces cerevisiae. FEMS Yeast Res 5:569–578
Esteban del Valle A, Aledo JC (2006) What process is glycolytic stoichiometry optimal for? J Mol Evol 62:488–495
Hardin G (1968) The tragedy of the commons. Science 162:1243–1248
Hauert Ch (2001) Fundamental clusters in spatial 2 × 2 games. Proc R Soc B 268:761–769
Hauert Ch, Doebeli M (2004) Spatial structure often inhibits the evolution of cooperation in the snowdrift game. Nature 428:643–646
Hauert Ch, Szabó G (2005) Game theory and physics. Am J Phys 73:405–414
Frick T, Schuster S (2003) An example of the prisoner’s dilemma in biochemistry. Naturwissenschaften 90:327–331
Kedem O, Caplan SR (1965) Degree of coupling and its relation to efficiency of energy conversion. Trans Faraday Soc 61:1897–1911
Kondepudi D, Prigogine I (1999) Modern thermodynamics. From heat engines to dissipative structures. Wiley, Chichester
Kreft J-U (2004) Biofilms promote altruism. Microbiology 150:2451–2760
Lawlor LR (1980) Overlap, similarity, and competition coefficients. Ecology 61:245–251
Leu J-YL, Murray AW (2005) Experimental evolution of mating discrimination in budding yeast. Curr Biol 16:280–286
MacLean RC, Gudelj I (2006) Resource competition and social conflict in experimental populations of yeast. Nature 441:498–501
Maddox J (1991) Is Darwinism a thermodynamic necessity? Nature 350:653
Maynard Smith J (1982) Evolution and the theory of games. Cambridge University Press, Cambridge
Nowak MA, May RM (1992) Evolutionary games and spatial chaos. Nature 359:826–829
Nowak MA, Sigmund K (2004) Evolutionary dynamics of biological games. Science 303:793–799
Pfeiffer T, Schuster S (2005) Game-theoretical approaches to studying the evolution of biochemical systems. Trends Biochem Sci 30:20–25
Pfeiffer T, Schuster S, Bonhoeffer S (2001) Cooperation and competition in the evolution of ATP-producing pathways. Science 292:504–507
Schweitzer F, Behera L, Mühlenbein H (2002) Evolution of cooperation in a spatial Prisoner’s Dilemma. Adv Comp Syst 5:269–299
Stucki JW (1980) The optimal efficiency and the economic degrees of coupling of oxidative phosphorylation. Eur J Biochem 109:269–283
Travisano M, Velicer GJ (2004) Strategies of microbial cheater control. Trends Microbiol 12:72–78
Velicer GJ, Lenski RE (1999) Evolutionary tradeoffs under conditions of resource abundance and scarcity: experiments with bacteria. Ecology 80:1168–1179
Voet D, Voet JG (2004) Biochemistry. Wiley, New York
Waddell TG, Repovic P, Meléndez-Hevia E, Heinrich R, Montero F (1997) Optimization of glycolysis: a new look at the efficiency of energy coupling. Biochem Educ 25:204–205
Warburg O (1956) On the origin of cancer cells. Science 123:309–314
Westerhoff HV, Hellingwerf KJ, van Dam K (1982) Thermodynamic efficiency of microbial growth is low but optimal for maximal growth rate. Proc Natl Acad Sci USA 80:305–309
Acknowledgments
We thank M. A. Medina for comments on the manuscript. We are grateful for financial support from the Universidad de Málaga and Grants CGL2007-65010 (J.C.A.) and CGL2004-016115/2005-01316 (J.A.P.-C.) from the Spanish Ministerio de Educación y Ciencia. J.A.P.-C. holds a research position at the Universidad de Málaga, funded by the Junta de Andalucía.
Author information
Authors and Affiliations
Corresponding author
Additional information
Reviewing Editor: Dr. Antony Dean
Appendices
Appendix A: Insight into the Thermodynamic Trade-off Between Output Power and Efficiency
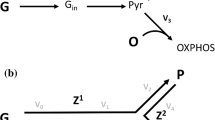
In the framework of nonequilibrium thermodynamics, glycolytic fluxes can be described by the following equations (Esteban del Valle and Aledo 2006):
where L ij ≥ 0 are phenomenological coefficients that incorporate kinetic (enzymatic) attributes. Equation (A2) can be interpreted as follows: glucose consumption is favored by its driving force (L22 X 2 > 0). In contrast, a high ATP concentration will lead to a very negative value of X 1, slowing down glucose consumption by a factor of L12 X 1. Therefore, when either X 1 = 0 or ATP synthesis and glucose breakdown are uncoupled (L12 = 0), the consumption of glucose becomes maximal and is given by J 2 = L22 X 2. Under these hypothetical conditions, the maximum attainable input power is L22 X 22 . It should be noted that the efficiency corresponding to this hypothetical situation is zero.
In order to stress the trade-off existing in glycolysis between the output power and the efficiency, the remainder of this section is devoted to express the output power as a function of the efficiency.
Bearing in mind the definition of output power and Eqs. (1), (A1), and (A2), we are entitled to write
This equation can be simplified taking into account some constraints that enforce certain relationships between their variables.
In this respect, an interesting concept introduced by Kedem and Caplan (1965) to assist in the study of linear energy converters is the degree of coupling, q = L12/(L11L22)1/2, which represents a dimensionless measure of how tightly the driven process is coupled to the driver process. Another useful concept is the so-called phenomenological stoichiometry, defined as Z = (L11/L22)1/2. Although Z must not be confused with the mechanistic stoichiometry, for values of q close to 1 the phenomenological and the mechanistic stoichiometries coincide (Stucki 1980). In the particular case of glycolysis, where ATP formation and glucose breakdown are tightly coupled processes, with a fixed stoichiometry of two molecules of ATP formed per molecule of glucose split, there are three constraints that need to be satisfied: (i) q = 1, (ii) J 1 /J 2 = 2, and (iii) Z = (L11/L22)1/2 = 2.
Bearing the first constraint in mind, L12 can be substituted for (L11L22)1/2. On the other hand, constraint (ii) allows us to rewrite Eq. (1) as –η/2 = X 1 /X 2.
When translating these substitutions into Eq. (A3) we obtain W o = ηX 22 ((L11L22)1/2 (–η/2) + L22), an expression that can be reorganized to yield W o = ηX 22 L22( (L11/L22)1/2 (–η/2) + 1), which can be further simplified when considering constraint (iii):
If the output power is normalized dividing by the maximum attainable input power, we obtain Eq. (2) from the main text.
Appendix B: Role of Entropy Production
In addition to W o and η, another relevant factor is the dissipation function or rate of entropy production, Φ, which can be interpreted as the rate at which the energy available for biological processes is reduced. This thermodynamic function can be formulated as the sum of the products of all the fluxes and their corresponding driving forces (Kondepudi and Prigogine 1999):
Herein, J 3 is the flux of all the ATP-consuming processes lumped together (Fig. 1A). These processes are driven by the hydrolysis of ATP, that is, X 3 = –X 1. Thus, J 3 = –L33 X 1 (Stucki 1980). Therefore, Eq. (B1) can be expressed as
According to the definitions given above, J 1 X 1 = –W o , J 2 X 2 = W o /η and X 1 = –ηX 2/2. Now, substituting into Eq. (B2), we obtain Ф = –W o + W o /η + L33 X 22 η2/4. When Eq. (A4) is taken in consideration, the dissipation function can be rewritten as
In order to assess the contribution of dissipative and efficient cells to the local entropy production, we need to know, in each case, the ratio L33/L44. To this purpose, Prigogine’s theorem of the minimum entropy production can be helpful. According to this theorem, in the steady state the entropy production must be minimal. In formal terms: dΦ/dη = 0 = 2(1 + L33/4L22) η – 2, from which we obtain the following constraint:
Herein, it may be convenient to remember that DMOP and EMOP organisms operate at efficiencies of 1/2 and 2/3, respectively. This means that L33 = 4L22 for dissipative cells, whereas in the case of efficient organisms the relation is L33 = 2L22. Bearing these considerations in mind, Eq. (B3) leads to a straightforward conclusion: the contributions of DMOP and EMOP cells to the local entropy production are different (ΦDMOP = 1/2 > ΦEMOP = 1/3).
Rights and permissions
About this article
Cite this article
Aledo, J.C., Pérez-Claros, J.A. & del Valle, A.E. Switching Between Cooperation and Competition in the Use of Extracellular Glucose. J Mol Evol 65, 328–339 (2007). https://doi.org/10.1007/s00239-007-9014-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-007-9014-z