Abstract
Background
The increasing popularity of minimally invasive aesthetic surgery has led to the ongoing development of new tissue fillers. These substances require a long-term safety assessment. One of the new fillers introduced to the market is a copolyamide-based gel called Aquafilling, subsequently renamed as Los Deline. In this review, we analyze the updated published information concerning this substance.
Methods
734 articles published within the last 20 years were retrieved from the databases of PubMed, Scopus, Embase, and Google Scholar. Thirty articles describing 251 patients met the inclusion criteria.
Results
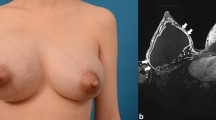
The most common complications were induration, firmness, pain, and gel migration. Twenty articles reported inflammation resulting from gel injection. The most common imaging method was MRI combined with USG. Six out of eight studies describing laboratory findings reported no elevation of CRP, WBC or ESR. The treatments with the lowest rates of reoperation were mastectomy and radical surgery (massive irrigation and infiltrating tissue excision). There was significant heterogeneity of pathogens obtained from gel-derived swabs.
Conclusions
Over time, Aquafilling injection can potentially result in a variety of complications. It triggers inflammation and prophylactic removal should be considered. MRI and USG are recommended in the diagnostic process. Elevated CRP, WBC, or ESR indicate the presence of comorbidity or advanced gel infection. The recommended treatment is radical surgery. There is not enough information available in the literature about reconstruction, especially regarding immediate reconstruction. Antibiotics should be administered according to the antibiogram, with ciprofloxacin and clindamycin being considered if empirical treatment is needed; however, further research is required.
Level of evidence: Not ratable







Similar content being viewed by others
References
Hedén P, Sellman G, von Wachenfeldt M, Olenius M, Fagrell D (2009) Body shaping and volume restoration: the role of hyaluronic acid. Aesthetic Plast Surg 33(3):274–282
Jin R, Luo X, Wang X et al (2018) Complications and Treatment Strategy After Breast Augmentation by Polyacrylamide Hydrogel Injection: Summary of 10-Year Clinical Experience. Aesthetic Plast Surg 42(2):402–409
Ono S, Ogawa R, Hyakusoku H (2010) Complications after polyacrylamide hydrogel injection for soft-tissue augmentation. Plast Reconstr Surg 126(4):1349–1357
Jung BK, Yun IS, Kim YS, Roh TS (2018) Complication of AQUAfilling® Gel Injection for Breast Augmentation: Case Report of One Case and Review of Literature. Aesthetic Plast Surg 42(5):1252–1256
Roh TS (2016) Position statement of Korean Academic Society of Aesthetic and reconstructive breast surgery: concerning the use of Aquafilling® for breast augmentation. Arch Aesthetic Plast Surg 22(01):45–46
Namgoong S, Kim HK, Hwang Y et al (2020) Clinical Experience with Treatment of Aquafilling Filler-Associated Complications: A Retrospective Study of 146 Cases. Aesthetic Plast Surg 44(6):1997–2007
Shin JH, Suh JS, Yang SG (2015) Correcting shape and size using temporary filler after breast augmentation with silicone implants. Arch Aesthetic Plast Surg 21:124–126
Gierej P, Radziszewski M, Miłoński P, Noszczyk B (2022) Distal Hand Migration of Polyacrylamide Gel after Breast Augmentation: A Case Report and Review of the Literature. Indian J Plast Surg 56(2):178–181
Chalcarz M, Żurawski J (2021) Injection of Aquafilling® for Breast Augmentation Causes Inflammatory Responses Independent of Visible Symptoms. Aesthetic Plast Surg 45(2):481–490
Bonnans C, Chou J, Werb Z (2014) Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15(12):786–801
Chalcarz M, Żurawski J (2022) The absence of early malignant changes in women subjected to Aquafilling breast augmentation on the basis of E-cadherin and N-cadherin immunohistochemical expression. Cent Eur J Immunol 47(4):350–356
Jung Choi Woo, Jin Song Woo, Gue Kang Sang (2023) Complications and management of breast augmentation using two different types of fillers: a case series. Arch Aesthetic Plast Surg 29(1):41–45
Ko KH, Jung HK, Park AY (2017) Radiologic features of distant filler migration with inflammatory reaction following augmentation mammoplasty using Aquafilling® filler. Iran J Radiol 14(4):e63468
Kim M, Kim J, Sun H, Yun J, Chung E (2022) Delayed purulent infected breast after a large-volume Aquafilling filler injection in an HIV-positive transgender patient: a case report. Arch Aesthetic Plast Surg 28(4):147–151
Nomoto S, Ogawa R (2021) Removal of Aquafilling Using Body-jet: A Waterjet-assisted Lipsuction Device. Plast Reconstr Surg Glob Open 9(6):e3451
Nomoto S, Hirakawa K, Ogawa R (2021) Safety of Copolyamide Filler Injection for Breast Augmentation. Plast Reconstr Surg Glob Open 9(2):e3296
Gierej P, Woźniak-Roszkowska E, Radziszewski M, Miszczyk J, Krześniak N, Noszczyk B (2023) Treatment of complications after minimally invasive breast augmentation with aquafilling gel. Aesthetic Plast Surg 47(6):2322–2329
Hwang YS, Byeon JY, Kim JH, Choi HJ, Oh MH, Lee DW (2023) Breast filler granuloma mistaken for implant rupture: A case report. Medicine (Baltimore) 102(22):e33785
Wünscher SV, Cambiaso-Daniel J, Gualdi A, Rappl T (2023) Long-term Complications of Gluteal Augmentation Using Aquafilling Filler: A Case Report. Indian J Plast Surg 56(3):267–269
Kim J, Chang H, Park JU (2019) Complication of Ruptured Poly Implant Prothèse® Breast Implants Combined with AQUAfilling® Gel Injection: A Case Report and Literature Review. Aesthetic Plast Surg 43(1):46–52
Loesch J, Eniste YS, Dedes KJ et al (2022) Complication after Aquafilling® gel-mediated augmentation mammoplasty—galactocele formation in a lactating woman: a case report and review of literature. Eur J Plast Surg 45:515–520
Elahi L, Ulrich F, Raffoul W, Rossi SA (2022) Management of a Large Quantity of Permanent Gluteal Copolyamide Fillers (Aqualift/Activegel): Literature Review and Algorithm. Aesthet Surg J Open Forum 4:ojac051
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Marcin Radziszewski conceived the idea and wrote the manuscript. Łucja Radziszewska and Zuzanna Kaczor collected and analysed the data. Piotr Gierej provided critical feedback and shaped the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Ethics approval is not applicable for this type of study.
Consent to participate
Consent to participate is not applicable for this type of study.
Consent to publish
Consent to publish is not applicable for this type of study.
Competing interests
The authors have no relevant financial or non-financial interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Radziszewski, M., Radziszewska, Ł., Kaczor, Z. et al. What do we know about Aquafilling tissue filler? – A systematic review. Eur J Plast Surg 47, 49 (2024). https://doi.org/10.1007/s00238-024-02197-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00238-024-02197-y