Abstract
Background
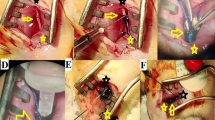
Fibrosis occurs during the healing process after nerve injury. Extraneural fibrosis occurs around the epineural sheath. Intraneural fibrosis occurs in the internal structure of the nerve. Prophylactic treatment with silicone gel sheets is effective in preventing severe scarring in the skin. Nerve action potential (NAP) recording is a well established, simple, and useful tool in the surgical treatment of nerve damage. Our aim in this study was to determine the effect of a silicone sheet on biophysical and histopathologic parameters when used in the treatment of peripheral nerve transection (neurotmesis) and crush injuries.
Methods
The study was conducted with 32 Wistar albino male rats. The rats in the neurotmesis and crush injury groups were divided into study and control groups. Before the injury and after 12 weeks, electrophysiological measurements were obtained. The sciatic nerve biopsy specimens were histopathologically evaluated.
Results
After 12 weeks, there was no statistical difference between pre- and post-injury ΔPP values in the PNR+SS group (p = 0.123). There was no statistically significant difference in post-injury ΔPP values between the CI and CI+SS groups (p = 0.201). The mean total histopathological score was significantly higher in the PNR+SS group than in the PNR group (p = 0.023).
Conclusions
In this rat model, a silicone sheet appears to have positive effects on peripheral nerve healing after primer repair and crush injury.
Level of evidence
Not gradable.




Similar content being viewed by others
References
Mustoe TA (2008) Evolution of silicone therapy and mechanism of action in scar management. Aesthet Plast Surg 32:82–92
Gold MH, Foster TD, Adair MA, Burlison K (2001) Lewis T Prevention of hypertrophic scars and keloids by the prophylactic use of topical silicone gel sheets following a surgical procedure in an office setting. Dermatol Surg 27:641–644
Quinn KJ, Evans JH, Courtney JM, Gaylor JDS (1985) Nonpressure treatment of hypertrophic scars. Burns 12:102–108
Wolfram D, Tzankov A, Pülzl P, Piza-Katzer H (2009) Hypertrophic scars and keloids: a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg 35:171–181
Zurada JM, Kriegel D, Davis IC (2006) Topical treatments for hypertrophic scars. J Am Acad Dermatol 55:1024–1031
Chang CC, Kuo YF, Chiu HC, Lee JL, Wong TW, Jee SH (1995) Hydration, not silicone, modulates the effects of keratinocytes on fibroblasts. J Surg Res 59:705–711
Yagmur C, Akaishi S, Ogawa R, Guneren E (2010) Mechanical receptor–related mechanisms in scar management: a review and hypothesis. Plast Reconstr Surg 126(2):426–434
Akaishi S, Akimoto M, Hyakusoku H, Ogawa R (2010) The tensile reduction effects of silicone gel sheeting. Plast Reconstr Surg 126(2):109e–111e
Atkins S, Smith KG, Loescher AR, Boissonade FM, O’Kane S, Ferguson MWJ, Robinson PP (2006) Scarring impedes regeneration at sites of peripheral nerve repair. Neuroreport 17:1245–1249
Wang ML, Rivlin M, Graham JG, Beredjiklian PK (2019) Peripheral nerve injury, scarring, and recovery. Connect Tissue Res 60(1):3–9
Tiel RL, Happel LT Jr, Kline DG (1996) Nerve action potential recording method and equipment. Neurosurgery 39(1):103–108
Alvites R, Caseiro AR, Pedrosa SS, Branquinho MV, Ronchi G, Geuna S, Varejão ASP, Maurício AC (2018) Peripheral nerve injury and axonotmesis: state of the art and recent advances. Cogent Medicine 5(1):1466404
Pan HC, Yang DY, Chiu YT, Lai SZ, Wang YC, Chang MH, Cheng FC (2006) Enhanced regeneration in injured sciatic nerve by human amniotic mesenchymal stem cell. J Clin Neurosci 13(5):570–575
Frostick SP (1995) The physiology and metabolic consequence of muscle denervation. Int Angiol 14:278–287
Ma HE, Omura T, Cobos EJ, Latrémolière A, Ghasemlou N, Brenner GJ, van Veen BL, Sawada T, Gao F, Coppola G, Gertler F, Costigan M, Geschwind D, Woolf CJ (2011) Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J Clin Invest 121:4332–4347
Mathieu L, Adam C, Legagneux J, Bruneval P, Masmejean E (2012) Reduction of neural scarring after peripheral nerve suture: an experimental study about collagen membrane and autologous vein wrapping. Chir Main 31:311–317
Ngeow WC (2010) Scar less: a review of methods of scar reduction at sites of peripheral nerve repair, Oral Surg. Oral Med Oral Pathol Oral Radiol Endod 109:357–366
Kaplan T, Kafa IM, Cansev M, Bekar A, Karli N, Taskapilioglu MO, Kanar F (2014) Investigation of the dose-dependency of citicoline effects on nerve regeneration and functional recovery in a rat model of sciatic nerve injury. Turk Neurosurg 24:54–62
Ozay R, Bekar A, Kocaeli H, Karli N, Filiz G, Ulus IH (2007) Citicoline improves functional recovery, promotes nerve regeneration, and reduces postoperative scarring after peripheral nerve surgery in rats. Surg Neurol 68:615–622
Sarhane KA, Ibrahim Z, Martin R, Krick K, Cashman CR, Tuffaha SH, Broyles JM, Prasad N, Yao ZC, Cooney DS, Mi R, Lee WA, Hoke A, Mao HQ (2019) Brandacher GMacroporous nanofiber wraps promote axonal regeneration and functional recovery in nerve repair by limiting fibrosis. Acta Biomater 88:332–345
Pleasure D, Bora FW, Lane J (1974) Prockop D Regeneration after nerve transection: effect of inhibition of collagen synthesis. Exp Neurol 45:72–78
Soller EC, Tzeranis DS, Miu K, So PT, Yannas IV (2012) Common features of optimal collagen scaffolds that disrupt wound contraction and enhance regeneration both in peripheral nerves and in skin. Biomaterials 33:4783–4791
Schaefer C, Sadosky A, Mann R, Daniel S, Parsons B, Tuchman M, Anschel A, Stacey BR, Nalamachu S (2014) Nieshoff EPain severity and the economic burden of neuropathic pain in the United States: BEAT Neuropathic Pain Observational Study. Clin Outcomes Res 6:483–496
Sauer SK, Bove GM, Averbeck B, Reeh PW (1999) Rat peripheral nerve components release calcitonin gene-related peptide and prostaglandin E2 in response to noxious stimuli: evidence that nervi nervorum are nociceptors. Neuroscience 92(1):319–325
Bove GM (2008) Epi-perineurial anatomy, innervation, and axonal nociceptive mechanisms. J Bodyw Mov Ther 12(3):185–190
Teixeira MJ, Almeida DB, Yeng LT (2016) Concept of acute neuropathic pain. The role of nervi nervorum in the distinction between acute nociceptive and neuropathic pain. Rev Dor São Paulo 17(1):5–10
Bove GM, Light AR (1995) Calcitonin gene-related peptide and peripherin immunoreactivity in nerve sheaths. Somatosens Mot Res 12:49–57
Schoonhoven R, Stegeman DF (1991) Models and analysis of compound nerve action potentials. Crit Rev Biomed Eng 19(1):47–111
Lee HJ (2001) Compound nerve action potential of common peroneal nerve recorded at fibular neck ıts clinical usefulness am. J Phys Med Rehabil Am J Phys Med Rehabil 80(2):108–112
Lim TK, Shi XQ, Johnson JM, Rone MB, Antel JP, David S, Zhang J (2015) Peripheral nerve injury induces persistent vascular dysfunction and endoneurial hypoxia, contributing to the genesis of neuropathic pain. J Neurosci 35(8):3346–33459
Gao Y, Weng C (2013) Changes in nerve microcirculation following peripheral nerve compression. Neural Regen Res 8(11):1041–1047
Saika T, Senba E, Noguchi K, Sato M, Yoshida S, Kubo T, Matsunaga T, Tohyama M (1991) Effects of nerve crush and transection on mRNA levels for nerve growth factor receptor in the rat facial motoneurons. Res Mol Brain Res 9(1-2):157–160
Yetiser S, Kahraman E (2008) An analysis of time-dependent changes of neurotrophic factors (BDNF, CNTF) in traumatic facial nerve ınjury of a nerve-cut and nerve-crush model in rats. Otol Neurotol 29(3):392–396
Gutmann E, Guteman L, Medawar PB, Young JZ (1942) The rate of regeneration of nerve. J Exp Biol 19:14–44
Haftek J, Thomas PK (1968) Electron-microscope observations on the effects of localized crush injuries on the connective tissues of peripheral nerve. J Anat 103(Pt 2):233–243
Niemi JP, DeFrancesco-Lisowitz A, Roldán-Hernández L, Lindborg JA, Mandell D, Zigmond RE (2013) A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration. J Neurosci 33(41):16236–16248
Shashkin P, Dragulev B, Ley K (2005) Macrophage differentiation to foam cells. Curr Pharm Des 11:3061–3072
Funding
Financial assistance was received from Sivas Cumhuriyet University Scientific Research Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving animal subjects were in accordance with the ethical standards of the institutional and/or national research committee. This study was also performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals. The Cumhuriyet University Ethical Comittee approved this study (decision No: 65202830-050 04 04-243).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Derebaşınlıoğlu, H., Demirkazık, A., Çiçek Doğan, İ. et al. The effect of a silicone sheet on sciatic nerve healing in rats. Eur J Plast Surg 46, 453–463 (2023). https://doi.org/10.1007/s00238-023-02071-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00238-023-02071-3