Abstract
Background
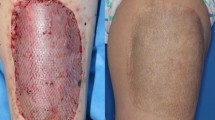
Full-thickness skin grafts (FTSG) are clinically utilised in settings to minimize contraction. However, FTSG contraction can have significant functional and aesthetical implications in reconstruction, particularly in head and neck cases and burns patients. This study was designed to establish a mouse model for studying the inhibitory effect of dermal substitutes on FTSG contraction. Secondarily, the model quantitatively assessed the specific effect of Integra™ on FTSG contraction and examined if Integra™ can be used in a single-stage procedure with a FTSG.
Methods
Eighteen male Wistar mice had a quadrangular 3 × 2 cm full-thickness skin defect produced on their back and divided into two groups. In the first group, the graft obtained from the defect was sutured back down as a FTSG. In the second group, Integra™ was placed under 30% of the FTSG in a grid pattern to allow vascular perfusion of the skin, in a single-stage procedure. Graft contraction in the two groups was accurately measured over a set time frame.
Results
The difference of wound contraction in week 1 was not statistically significant; however, the differences increased dramatically after week 1 as the Integra™ was vascularized. This difference became statistically significant at week 2 (p < 0.05), week 3 (p < 0.01) and week 4 (p < 0.01). By week 3, a significant 17% difference in graft contraction was seen and the difference in contraction remained stationary in week 4 (at 18%).
Conclusions
The application of a split dermal template resulted in a significant decrease in FTSG contraction. Additionally, Integra™ was observed to be successfully utilised with a FTSG in a single-stage procedure. This technique could be utilised in an effort to reduce graft contraction in complicated sites.
Level of evidence
All experimental studies are not ratable.


Similar content being viewed by others
References
Stephenson AJ, Griffiths RW, La Hausse-Brown TP (2000) Patterns of contraction in human full thickness skin grafts. Br J Plast Surg 53:397–402
Stekelenburg CM, Simons JM, Tuinebreijer WE, van Zuijlen P (2016) Analyzing contraction of full thickness skin grafts in time: choosing the donor site does matter. Burns 42(7):11
Hayashida K, Akita S (2017) Surgical treatment algorithms for post-burn contractures. Burns Trauma 5:9
Nguyen DQ, Potokar TS, Price P (2010) An objective long-term evaluation of Integra (a dermal skin substitute) and split thickness skin grafts, in acute burns and reconstructive surgery. Burns 36:23–28
Van Zuijlen P, Van Trier A, Vloemans J et al (2000) Graft survival and effectiveness of dermal substitution in burns and reconstructive surgery in a one-stage grafting model. Plast Reconstr Surg 106(3):615–623
Leibovitch I, Huilgol SC, Hsuan JD, Selva D (2005) Incidence of host site complications in periocular full thickness skin grafts. Br J Ophthalmol 89(2):219–222
O’Connor FE, Frew Q, Din A et al (2015) Periorbital burns–a 6 year review of management and outcome. Burns 41:616–623
Van Zuijlen P, Gardien K, Jaspers M et al (2015) Tissue engineering in burn scar reconstruction. Burns Trauma. 3:18
Chu C, Mc Manus A, Matylevich N et al (2002) Integra as a dermal replacement in a meshed composite skin graft in a rat model: a one-step operative procedure. J Trauma 52:122–129
Böttcher-Haberzeth S, Biedermann T, Schiestl C, Hartmann-Fritsch F, Schneider J, Reichmann E, Meuli M (2012) Matriderm® 1 mm versus Integra® Single Layer 1.3 mm for one-step closure of full thickness skin defects: a comparative experimental study in rats. Pediatr Surg Int 28:171–177
Dunn L, Prosser HC, Tan JT, Vanags LZ, Ng MK, Bursill CA (2013) Murine model of wound healing. J Vis Exp 75:e50265
Ericsson AC, Crim MJ, Franklin CL (2013) A brief history of animal modeling. Mo Med 110:201–205
Heimbach DM, Warden GD, Luterman A, Jordan MH, Ozobia N, Ryan CM, Voigt DW, Hickerson WL, Saffle JR, DeClement F, Sheridan RL, Dimick AR (2003) Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J Burn Care Rehabil 24(1):42–48
Rashid OM, Nagahashi M, Takabe K (2012) Management of massive soft tissue defects: the use of INTEGRA® artificial skin after necrotizing soft tissue infection of the chest. J Thorac Dis, J Thorac Dis 4(3)
Stekelenburg CM, van der Wal MB, Knol DL, de Vet HC, van Zuijlen PP (2013) Three-dimensional digital stereophotogrammetry: a reliable and valid technique for measuring scar surface area. Plast Reconstr Surg 132:204–211
Hansen AL, Voigt DW, Wiebelhaus P, Paul CN (2001) Using skin replacement products to treat burns and wounds. Adv Skin Wound Care 14:37–44
Chua AC, Khoo YC, Tan BK et al (2016) Skin tissue engineering advances in severe burns: review and therapeutic applications. Burns Trauma 4(1):3
Ruszczak Z (2003) Effect of collagen matrices on dermal wound healing. Adv Drug Deliv Rev 55:1595–1611
Philp L, Umraw N, Cartotto R (2012) Late outcomes after grafting of the severely burned face: a quality improvement initiative. J Burn Care Res 33:46–56
Burke JF, Yannas IV, Quinby WC Jr, Bondoc CC, Jung WK (1981) Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg 194:413–428
Rathore DS, Chickadasarahilli S, Crossman R, Mehta P, Ahluwalia HS (2014) Full thickness skin grafts in periocular reconstructions: long-term outcomes. Ophthalmic Plast Reconstr Surg 30(6):517–520
Truong AT (2005) Comparison of dermal substitute in wounds healing utilizing a nude mouse model. J Burns Wounds 14:72–82
Philandrianos C, Andrac-Meyer L, Mordon S, Feuerstein JM, Sabatier F, Veran J, Magalon G, Casanova D (2012) Comparison of five dermal substitutes in full-thickness skin wound healing in a porcine model. Burns. 38:820–829
Burd A, Wong P (2010) One-stage Integra reconstruction in head and neck defects. J Plast Reconstr Aesthet Surg 63:404–409
Hur GY, Seo DK, Lee JW (2014) Contracture of skin graft in human burns: effect of artificial dermis. Burns 40(8):12
Jacoby SM, Bachoura A, Chen NC, Shin EK, Katolik LI (2013 Sep) One-stage Integra coverage for fingertip injuries. Hand 8(3):291–295
Reid M, Currie L, James S, Shape J (2007) Effect of artificial dermal substitute, cultured keratinocytes and split thickness skin graft on wound contraction. Wound Repair Regen 16:889–896
Seo DK, Kym D, Hur J (2014) Management of neck contractures by single-stage dermal substitutes and skin grafting in extensive burn patients. Ann Surg Treat Res 87(5):253–259
Funding
This work was supported by a grant from the ANZAC Research Institute, Concord Repatriation General Hospital, Sydney, Australia (NHMRC grant GNT9000309).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Charles Meares, Vlad Illie, Li Zhe, and Peter Maitz declare that they have no conflict of interest.
Ethical approval
Approval obtained from the ANZAC Research Institute and all animal procedures were performed in accordance with the guidelines issued by the Intramural Research Program of the National Institutes of Health protocol number 444TGB2016
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meares, C., Illie, V., Zhe, L. et al. A novel technique of reducing full-thickness skin graft contraction using a dermal substitute: an animal model study. Eur J Plast Surg 43, 535–540 (2020). https://doi.org/10.1007/s00238-020-01661-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00238-020-01661-9