Abstract
Tocilizumab is a biological immunosuppressive drug used in the treatment of psoriasis arthritis. It works by blocking the interleukin 6 receptor and therefor blocking the immune response caused by IL-6 which plays an important role in arthritis. Tocilizumab is commonly used in RA patients who either have experienced insufficient effect of other treatment options or who have had unacceptable side effects from previous treatment. With this case report, we would like to raise awareness of a potentially previously unheard of adverse effect of tocilizumab treatment on split thickness skin graft (STSG). We present a case of a 61-year-old man, treated with tocilizumab for severe poly-articular, erosive psoriasis arthritis. He was diagnosed with BCC on the scalp and underwent excision followed by STSG with no complications. The patient experienced graft loss after commencing tocilizumab treatment days to weeks post grafting on numerous occasions. We find this possible adverse effect of tocilizumab on skin grafts to be of great importance to report as it is not previously mentioned in any literature. We hope that this case report will increase the awareness of this possible adverse effect on STSG in patients treated with tocilizumab (TCZ). As the patient is dependent on arthritis symptoms to be well controlled, it is of great importance to both medical and surgical teams responsible for treatment to be able to collaborate to plan best treatment, timing, and strategy.Level of Evidence: Level V, risk / prognostic study
Level of Evidence: Level V, risk / prognostic study.
Similar content being viewed by others
Introduction
Tocilizumab (RoActemra® - Roche A/S, Hvidovre, Denmark) is a biological immunosuppressive drug used in the treatment of psoriasis arthritis. It is a humanized monoclonal antibody that works by blocking the interleukin 6 receptor and therefor blocking the immune response caused by IL-6 which plays an important role in arthritis [1, 2]. Tocilizumab is commonly used in RA patients who either have experienced insufficient effect of other treatment options or who have had unacceptable side effects from previous treatment. It can be used as monotherapy or in combination with methotrexate. There is no significate difference in the clinical effect if used as mono or combination therapy, but patients treated with combination therapy have significantly less progression of joint erosion on radiological examination [2].
The well-documented, very common side effects of tocilizumab include neutropenia and upper airway infections (> 10% of patient population). Common side effects affecting the skin have been reported as skin infection, pruritus, hives, and skin rash (1–10% of patient population) [2].
We present a case of a 61-year-old male, treated with tocilizumab for severe poly-articular, erosive psoriasis arthritis. He was diagnosed with basal cell carcinoma (BCC) on the scalp and underwent excision followed by split thickness skin grafting (STSG) with no complications. The patient then experienced multiple graft losses after commencing tocilizumab treatment days to weeks post operatively.
Case report
A 61-year-old male known with severe erosive psoriasis arthritis was diagnosed with BCC on his scalp in October 2008. He was successfully treated with excision and STSG, with no post-operative complications and a fully healed graft. At that time, the patient was undergoing anti immune modeling treatment and antineoplastic therapy for his arthritis with Humira (adalimumab) and methotrexate (Table 1).
In September 2009, a year after initial surgical treatment, the patient experienced recurrence of the BCC in the graft and surrounding areas. Due to his severe arthritis, he was unable to stop his immune modeling medicine to receive surgical treatment and the decision was made to treat him with photodynamic therapy (PDT) and curetting of the carcinomas. He received six PDT treatments which resulted in a further ulcer in the treated area in the periphery of the STSG.
Due to progressing erosive radiological changes in his joints, it was decided to change medical treatment from TNF alfa inhibitors to tocilizumab in November 2011. The patient was still experiencing too severe symptoms from his arthritis to be able to stop immunosuppressive treatment for surgery and the next treatment of choice was radiation therapy (RT) which was completed in July 2013. After finished RT treatment, the patient was left with an ulcer on his scalp of approximately 2.5 × 7 cm. Due to his medical condition, he was treated conservatively with ointment (Løhr) to promote granulation. This was the case for 14 months.
In September 2014, the patient’s arthritis symptoms were well enough controlled for the patient to comply to an attempt to treat the defect with a STSG. Tocilizumab was stopped prior to surgery. The graft failed 2 weeks post operatively, while still off tocilizumab treatment, due to suspicion of skin infection and the patient was left with exposed bone on his scalp. The decision was made to try re-grafting the defect, and in 2015, a two-staged surgery was performed. The first stage with bone chiseling was performed in February, followed by STSG in April 2015 which fully healed with no post-operative complications. During this time, from the beginning of February until June 2015, the tocilizumab treatment was stopped. When treatment was restarted in June 2015, the patient developed a defect in the STSG. Due to again experiencing severe arthritis symptoms, conservative treatment with granulation-promoting ointment was the only realistic option.
In December 2015, the patient was admitted to hospital for a severe infection caused by a subdural empyema under the exposed bone area. Tocilizumab was stopped on admission due to the underlying infection. The patient underwent joint neuro and plastic surgery treatment in February 2016 with evacuation of the empyema, a visor flap, and a STSG to cover the secondary defect. Unfortunately, the graft failed 4 weeks post-operatively due to what was interpreted as clinical infection. Again, the patient was treated conservatively with cod liver oil ointment to promote wound healing. Tocilizumab treatment was paused for 14 months from admission and restarted in March 2017.
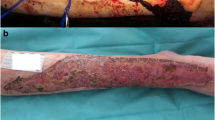
In August 2017, the patient was again medically fit for surgery. Tocilizumab was stopped 3 weeks pre-operatively and surgery was performed with bone chiseling and a STSG as a single-stage procedure. The surgery was successful with 100% take of the STSG on POD 7 on dressing removal (Figs. 1 and 2). Tocilizumab was restarted 14 days post operatively, and at clinic appointment 4 weeks post operatively, the graft was 100% healed and the patient was discharged from further follow-up. In December 2017, an extra clinic appointment was made as the graft yet again had started to dissolve, 12 weeks after re-starting tocilizumab (coinciding with a superficial skin infection of the left upper arm). On clinical inspection, approximately 65% of the graft was left (Fig. 3). The patient was at this point again experiencing severe arthritic pains and conservative treatment with ointment to stimulate granulation was again begun.
Discussion
Infection is the most common adverse effect of tocilizumab (TCZ), given its mode of action. Skin and subcutaneous infections were reported with a higher frequency in the TCZ (4.1%) vs. methotrexate group (0.7%) by G. Jones et al. in their review [3]. Nishimoto et al. reported skin rash as a mild or moderate adverse event in 6.6% of their study population treated with TCZ as monotherapy [4]. In their 5-year extension study, Nishimoto et al. also reported that none of the skin infections seen were severe adverse effects, and no patients included in either of their mentioned studies experienced skin graft failure, skin graft infection, or other skin graft-related problems [5].
J. S. Smolen et al. reported 13% of patients receiving 4 mg/kg and 18% of patients receiving 8 mg/kg to experience adverse effects in form of “any skin and subcutaneous disorder” compared to placebo group of 7%. Of these, rash made up 6% and 5% in the two groups compared to 1% in the placebo group. Again, it is not here further specified if the patients included experienced adverse effects on skin grafts as part of the “any skin and subcutaneous disorder” category [6].
Our patient experienced graft loss, either partial or complete, on several occasions all correlating to Tocilizumab treatment. Three of these were in direct relation to restarting treatment post operatively where the graft failed within weeks. The fourth occasion was the first attempt at grafting the patient after initially starting tocilizumab treatment. The graft failed before tocilizumab was re-started post operatively due to infection, but it cannot be ruled out that tocilizumab had an influence on the healing process or was the underlying cause of the infection due to its mode of action. It is plausible that other factors such as the recurring infections elsewhere (empyema, respiratory infections) might have had an impact on the patient’s immune response system and healing ability. The patients’ all over health, nutritional state, stress factors, competing immune illness, and infections may also have contributed to graft loss [7]. A biopsy of the grafted area/wound could be considered if the patient experiences additional graft loss to further rule out an alternative underlying pathophysiology.
Nowhere, to our knowledge, in the current literature is tocilizumab reported to affect skin in a degenerative or erosive way and no reports of adverse side effects on skin grafts have been reported. This may be due to the small number of patients with moderate to severe arthritis undergoing simultaneous tocilizumab treatment and surgical skin grafting.
Another important observation is the correlation between the severity of the patient’s arthritic symptoms and the timings of the surgical procedures. A repetitive cycle is clearly noticeable in the fact that our patient could only undergo surgical treatment when his overall well-being and health allowed him to (Fig. 4). This was on every occasion weeks to months after tocilizumab treatment was begun and his arthritis symptoms were well controlled. This only stresses the importance of collaboration between treating medical physicians and the aspect of timing surgical intervention when medical symptoms are well controlled, as one cannot go without the other when taking all patient factors in to consideration.
It is likely to assume that the skin graft failure on numerous occasions is a direct cause of tocilizumab treatment, although we are unable to explain cause and effect, but coincidence being unlikely due to the number of surgical attempts undertaken.
Conclusion
As the patient is dependent on arthritis symptoms to be well controlled, it is of great importance to both medical and surgical teams responsible for treatment to be able to collaborate to plan best treatment, timing, and strategy. We find this possible adverse effect of tocilizumab on skin grafts to be of great importance to report as it is not previously mentioned in any literature. We hope that this case report will increase the awareness of this possible adverse effect on STSG in patients treated with TCZ.
References
Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ, Siri DA, Tomsic M, Alecock E, Woodworth T, Genovese MC (2010) Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis 69(1):88–96
Mogens Pfeiffer Jensen, K.S.-P. Interleukin-6-hæmmere (biologiske antireumatika). 2017 07.03.2017; Available from: https://pro.medicin.dk/Laegemiddelgrupper/grupper/317923
Jones G, Ding C (2010) Tocilizumab: a review of its safety and efficacy in rheumatoid arthritis. Clin Med Insights Arthritis Musculoskelet Disord 3:81–89
Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J, Kishimoto T (2009) Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol 19(1):12–19
Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J (2009) Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis 68(10):1580–1584
Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, Woodworth T, Alten R (2008) Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 371(9617):987–997
Guo S, Dipietro LA (2010) Factors affecting wound healing. J Dent Res 89(3):219–229
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This case report was written in accordance with the guidelines and rules for publication and was following the COPE guidelines stated in the “Instructions for Authors” section. Ethical approval was obtained from the Ethical Board of the department.
Conflict of interest
Matilda Svenning and Christian Bonde declare that they have no conflict of interest.
Patient consent
Informed consent for inclusion was obtained from the patient included in the study. Additional informed consent was obtained from the individual participant for whom identifying information, including photographs, is included in this article.
Funding
There was no funding received by any of the named authors.
Rights and permissions
About this article
Cite this article
Svenning, M.I., Bonde, C. Adverse effect of tocilizumab treatment on split thickness skin graft—a case report. Eur J Plast Surg 42, 101–104 (2019). https://doi.org/10.1007/s00238-018-1451-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00238-018-1451-y