Abstract
Background
Matrices are now commonly used in breast reconstruction, but the scientific evidence is still scares. The main aim was to compare complications and the need for corrections in immediate breast reconstruction with the porcine-derived Surgisis® with the traditional muscle-covered technique. The secondary aim was to compare long-term quality of life and satisfaction.
Methods
All consecutive patients who had their breast reconstructed with a Surgisis® or muscle-covered tissue expander/implant were included. Patients were followed clinically and with BREAST-Q.
Results
During the study period, 116 reconstructions (71 patients) were operated in the Surgisis® group and 132 reconstructions (90 patients) in the control group. The median follow-up time was 74 months (min 43–max 162). The total early complication rate was 37% in the Surgisis® group and 27% in the control group. There were no differences in implant loss (p = 0.68) or total number of complications (p = 0.24) between the two groups. Risk factors for complications were mainly patient characteristics and the use of a tissue expander. There was a slightly higher capsular contracture frequency in the Surgisis® patients (4.2% vs. 2.5%). The need for corrections and patient satisfaction and quality of life were similar in the two groups.
Conclusions
The use of Surgisis® in implant-based reconstruction seems to result in an acceptable total early complication rate. The rate might be higher in tissue expander-based reconstruction. Risk factors are mainly patient characteristics. The capsular contracture rate and need for corrections, as well as patient satisfaction and quality of life, are similar in the Surgisis® patients and muscle-covered controls.
Level of evidence: III
Similar content being viewed by others
Introduction
In modern implant-based breast reconstruction, it has become common to use matrices and meshes, such as acellular dermal matrices (ADMs). Often stated advantages [1] include a better control and definition of the implant pocket and inframammary fold and the possibility to use a dual-plane technique and less muscle dissection [2,3,4]. Moreover, capsule formation may be less pronounced when matrices are used [2, 5]. Possible drawbacks are an increased risk of complications, such as infection, skin necrosis, loss of implant and seroma formation [6, 7] and a non-negligible cost [8].
The usage of matrices was first reported in secondary aesthetic breast surgery in 2001 [9] and in breast reconstruction in 2005 [10]. Reports have stated that the majority of plastic surgeons in the USA now use a matrix in implant-based breast reconstruction [1]. There are many different matrices currently on the market, including human-derived (e.g. AlloDerm®), porcine-derived (e.g. Permacol™, Strattice®) and bovine-derived (e.g. Veritas®) matrices. However, most of the supporting evidence for ADMs is based on case series and retrospective reviews and the difference in outcomes between the various matrices is unclear [1, 11].
Surgisis® (Cook Inc., West Lafayette, USA) is a sterile, biological porcine-derived dried matrix composed of a multilayered non-cross-linked collagen (types I, III and V), glycosaminoglycans, proteoglycans, glycoproteins and growth factors. It is produced from the small intestine submucosa, is biodegradable and works as an acellular scaffold that is incorporated and neovascularised in animal models and in humans [12,13,14,15,16]. This is the first study on the usage of Surgisis® as an adjunct in breast reconstruction in a larger series.
The main aim of this study was to compare short- and long-term (> 90 days) complications and predictors for complications in immediate tissue expander (TE)/implant-based breast reconstruction and Surgisis® with the traditional muscle-covered technique. The secondary aim was to compare patients’ and controls’ long-term quality of life and satisfaction.
Patients and methods
All consecutive patients who had their breast reconstructed with Surgisis® (small intestine submucosa (SIS)) and a tissue expander or a permanent implant between 2005 and 2014 in our department were prospectively included in the study. As a control group, all consecutive patients operated on with a muscle-covered TE/implant in the same time period were included. Inclusion criteria were indication for a unilateral or bilateral mastectomy, for either oncological or prophylactic reasons, and an immediate breast reconstruction. Exclusion criterion was the inability to give informed consent. Indication and operation technique were discussed at an MDT conference in all cases. Procedures followed were in accordance with the Helsinki Declaration of 1964, as revised, and the Good Clinical Practice (GCP) guidelines.
Surgical technique
Preoperatively, with the patient in a sitting position, the planned incision pattern, the position of the future implant pocket and the anatomic boundaries of the breast were marked. In ptotic breasts, a Wise-pattern skin resection was made; otherwise, a submammary incision was performed. In cases with previous surgical scars, modified skin patterns were used depending on scar position. During the surgery, a breast surgeon carried out the mastectomy. A plastic surgeon then created a submuscular pocket, releasing the inferior-medial and the inferior attachments of the major pectoral muscle. Surgisis® (SIS) (Cook Medical, Bloomington, IN, USA) was sutured, with 2-0 Maxon™ (Covidien, Ireland, Dublin), to the inferior border of the pectoral muscle and to the chest wall corresponding to the inframammary fold and lateral border of the implant pocket. In the control group, the serratus muscle was raised in combination with the major pectoral muscle to cover most of the lateral aspect of the TE/implant. Implant size was determined with a sizer, and either an anatomical TE (CPX®; Mentor Worldwide LLC, CA, USA) or a permanent anatomical silicone implant (direct-to-implant, DTI) (CPG®; Mentor Worldwide LLC, CA, USA) was placed into the submuscular pocket. TEs were partially filled at the end of surgery, taking care to achieve a tensionless closure. A submuscular drain and a subcutaneous suction drain were placed in each breast and were kept in place until the output was less than 30 ml per 24 h. Patients received prophylactic perioperative intravenous antibiotics in the form of cloxacillin, or clindamycin in case of allergy, and orally postoperatively until the drains were removed. In general, the patients were hospitalised for 48 h. TEs were exchanged for a permanent implant 3–6 months after the first operation.
Clinical data and follow-up protocol
Registered clinical data were age, preoperative body mass index (BMI, body weight in kilograms divided by the square of body height in meters), tobacco use and comorbidities. Factors related to the surgery, that is indication for mastectomy, type of tissue expander or implant and any pre- or post-reconstruction radiotherapy, were also recorded.
Patients were evaluated clinically and 1 week, 3 months and 1 year postoperatively. After that, patients were evaluated clinically in case they experienced any problems. A clinical case report form (CRF) was used to ensure that all patients were evaluated in a standardised fashion. The complications investigated were based on complications previously described in immediate breast reconstruction [17]. Short-term complications (< 90 days) were divided into major and minor complications. Major complications were implant loss (including previous implant exposure, mesh exposure, implant loss and infection), re-operation, and thromboembolic events. Minor complications were seroma formation requiring aspiration, hematoma not requiring re-operation, type IV delayed hypersensitivity reactions (“red breast”), and epidermolysis/minimal wound rupture/necrosis not requiring revision. Long-term complications (> 90 days) were defined as all operations and corrections.
Patient satisfaction and quality of life
Patients’ satisfaction and well-being were measured with the Swedish version of the postoperative reconstruction module of BREAST-Q [18, 19]. The BREAST-Q was developed to measure quality of life in breast patients. It has been validated [18, 19], and the translation process to Swedish was performed according to guidelines for linguistic validation of patient-reported outcome instruments [20]. Only domains relevant for the aim of the study were analysed: Quality of life domains: (1) psychosocial well-being, (2) sexual well-being and (3a) physical well-being (chest and upper body) and Satisfaction domains: (1) satisfaction with breasts and (5) satisfaction with outcome. The questionnaire was sent by post to the subjects and controls with an explanatory letter and return envelope. A remainder was sent after 2 weeks to those who had not returned the questionnaire. The first round of questionnaires was sent to the patients in median 74 months (min 43–max 162) after the operation.
Statistics
Categorical variables were described by number and percentage and continuous variables by mean, standard deviation, median, minimum and maximum. For test of differences between the two groups, Fisher’s exact test was used for dichotomous variables, the chi-square test for non-ordered categorical variables and the Mann-Whitney U test for continuous variables. The relation between two ordered categorical variables was tested by the Mantel-Haenszel chi-square test.
The prediction of complications during the study with baseline characteristic variables was performed by using logistic regression. Odds ratio (OR) and 95% confidence intervals (CIs) were presented from these analyses with associated p value, area under the ROC curve as goodness-of-fit statistics and probability plots for graphical presentation. For continuous variables with an area under the ROC curve of > 0.70, the cut-off maximising sensitivity and specificity was identified and a dichotomised variable based on this cut-off was also analysed using logistic regression. All analyses were performed separately for permanent implants and tissue expanders.
All tests were two-tailed and conducted at a 0.05 significance level. All analyses were performed by using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
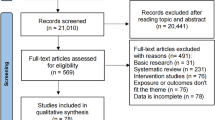
During the study periods, 248 breasts were reconstructed, and 116 reconstructions (71 patients, 26 unilateral and 45 bilateral reconstructions) were performed in the Surgisis® group and 132 reconstructions (90 patients, 48 unilateral and 42 bilateral reconstructions) in the control group. Most of the mastectomies were prophylactic (70%), and about half of the patients were operated on bilaterally. There were no differences in demography and reason for surgery between the groups. Details for patients reconstructed with implants and TEs, respectively, in each group are given in Table 1. The median follow-up time was 68 months (min 43–max 158) in the Surgisis group and 100 months (min 44–max 162) in the control group.
Early complications (< 90 days)
The overall complication rate was 37% (26/71) in the Surgisis® group and 27% (24/90) in the control group. In the Surgisis® group, there were 12/71 (17%) major complications (all implant losses) and 14/71 minor complications (20%). In the control group, there were 12/90 (13%) major complications (all implant losses) and 12/90 (13%) minor complications. One patient in the Surgisis® group and three patients in the control group who were reconstructed bilaterally lost implants on both sides. The overall frequency of the number of breasts with implant loss was 11% in both groups (13/116 and 15/132). There were no differences on patient level in implant loss (p = 0.68) or total number of complications (p = 0.24) between the two groups. Complication frequencies for patients reconstructed with implants and TEs, respectively, in each group are given in Table 2. In brief, there was a difference between Surgisis® patients and controls as regards overall complication rate in patients reconstructed with TEs (p = 0.0056), but no other differences between the groups.
In the Surgisis® group as a whole, significant predictors for developing any complication were radiation (OR (95% CI) = 3.10, p = 0.036) (Fig. 1) and BMI (OR = 4.1, p = 0.0094) (Fig. 2). In the implant subgroup, the only statistically significant predictor for developing any complication was a higher BMI (p = 0.0094). Moreover, there was a higher complication frequency in smokers (67%) than in non-smokers (25%) (p = 0.15) (Table 3). Smoking was a risk factor for implant loss specifically (p = 0.025). In the TE subgroup, significant predictors (Table 4) for developing any complication were Surgisis® (p = 0.034) and radiation (p = 0.036). Radiation was a risk factor for implant loss specifically (p = 0.058).
There were no early complications following the exchange of TEs to permanent implants in patients reconstructed in two stages.
Long-term follow-up
Among the patients included in the early years, all controls, nine women (9/90, 10%) had died from metastatic breast or ovarian cancer. Therefore, a clinical long-term follow-up was performed for 81 controls. There was a slightly higher capsular contracture frequency in the Surgisis® patients (4.2%) than in the controls (2.5%). The most common corrections performed in both groups were lipofilling and correction of implant position (Table 5).
Patient satisfaction and quality of life
Completed questionnaires were received from 49/71 (69%) of the Surgisis® patients and from 55/81 (68%) of the controls. There were no differences between the groups as regards satisfaction and quality of life, as measured with BREAST-Q (Table 6).
Discussion
Biological matrices have been used in breast reconstruction since 2005 [10] and are now used by the majority of plastic surgeons in the USA [1]. There are several different matrices currently on the market, and most of the supporting evidence is based on case series and retrospective reviews [1, 11]. This is the first study on the usage of Surgisis® (Cook Medical, Bloomington, IN, USA), a porcine-derived acellular matrix, as an adjunct in breast reconstruction.
It has been postulated that the use of matrices in breast reconstruction might increase the risk of complications as they constitute a non-vascularised material in a setting with poorly circulated mastectomy flaps [6, 7]. Indeed, the total early complication rate in this study was considerably higher than that in previously published studies involving the use of biologic matrices [21]. However, in previous studies, it is often unclear how the different complications were defined, which complications were included and how they were diagnosed [11]. Nonetheless, the high total complication frequency for Surgisis® is noteworthy and further studies on the safety of Surgisis® are needed before it is widely adopted in breast reconstruction.
The ultimate complication of a breast reconstruction is an implant loss, most often caused by implant infection. A previous in vitro study has shown that Surgisis® has a higher bacterial load than other polymer materials, when incubated with Staphylococcus [22], which might indicate that it should be avoided in contaminated surgical fields. Similarly, a clinical study on hernia repair has indicated that the frequency of wound infections could be high when Surgisis® is used [14]. Nonetheless, a meta-analysis performed in our department [11] did not support an association between matrices and meshes and implant loss. Those findings are supported by the results of this study, as a higher incidence of implant loss could not be seen in the Surgisis® group than in the control group. In summary, our findings do not support that the use of Surgisis® leads to more frequent implant losses.
Nonetheless, it is worth noting that the overall complication rate as well as implant loss frequency among Surgisis® patients was considerably higher when a tissue expander was used than when an implant was used (47% vs. 26% and 25% vs. 8.6%, respectively, Table 2). Contrary to our findings, previous research has indicated that single-stage reconstruction might be a risk factor for complications when matrices/meshes are used [21]. As the use of two-stage breast reconstruction with matrices/meshes sometimes might be indicated [23] and is widely used [24], further studies on single-stage vs. two-stage reconstruction are warranted.
A few clinical characteristics, such as a BMI over 30, smoking and large breasts (> 600 g), have previously been identified as risk factors for complications in breast reconstruction with matrices [25]. The same tendencies, regarding BMI and smoking, could be seen in our study (Fig. 2, Tables 3 and 4). However, the risk increase with smoking might be understated in this study as smoking is considered a contraindication for breast reconstruction in Sweden. Therefore, there might be a few patients in the study who have stated that they have stopped smoking even though they have not. Similarly, a BMI over 30 is a contraindication and, hence, there are few patients pertaining to this group in this study, which makes the statistical analysis difficult. In brief, the risk factors for complications seem to be similar in Surgisis® and in other matrices.
Radiation therapy is an important risk factor that may influence the result of breast reconstruction in both the short and long term. For example, Spear et al. reported a 45.5% complication rate in irradiated breasts compared to 4.3% in non-irradiated breasts reconstructed with AlloDerm® [2]. In the present study, it is noticeable that the frequency of implant/TE losses was higher in irradiated Surgisis® patients than in non-irradiated Surgisis® patients (40% vs. 11%). Only 15 Surgisis® patients received radiation, but the findings indicate that Surgisis® should be used with caution in patients who have or will receive radiation. Further studies are needed on radiotherapy in the context of matrices.
Often stated advantages [1] with the use of matrix are a better aesthetic result, with a better control and definition of the implant pocket and inframammary fold, and the possibility to use a dual-plane technique and less muscle dissection [2,3,4]. However, it is difficult to evaluate the aesthetic result after breast reconstruction, as there are no validated instruments available and no consensus on how the evaluation should be performed [11]. In the present study, long-term patient satisfaction and quality of life, as measured with BREAST-Q, were similar in the Surgisis® patients and muscle-covered controls (Table 6). This indicates that the clinical difference in aesthetic results might not be significant between matrix and no matrix.
Previously, it has been demonstrated that the surface of human matrix (AlloDerm®) stretches in breast reconstruction [26]. The elastic properties make it difficult to use the right size of matrix and surgical technique to achieve the best aesthetic result and avoid bottoming out [26]. An animal study on Surgisis® has shown that it stretches significantly after implantation, with an average area increase of 36% (26% in width and 17% in length) [26]. . Nonetheless, in this study, the long-term frequency of corrections were not higher in the Surgisis® patients than in the controls (Table 5), contradicting a clinically significant stretch over time.
Another stated advantage of matrices is a lower frequency of capsular contracture [11]. However, the scientific evidence for this is low [11]. In our study, the frequency of capsular contracture was actually higher in the Surgisis® group than in the controls (4.2% vs. 2.5%). Indeed, similar findings have been seen in some previous studies (e.g. [27, 28]). In brief, the scientific evidence for the hypothesis that meshes give a lower capsular frequency is still weak and not supported by our study.
The present study has a few scientific weaknesses. Firstly, the patients were not randomised to Surgisis® or muscle coverage. A consecutive sampling technique was used; that is, all consecutive patients, fulfilling the inclusion criteria, during a time frame were allocated to Surgisis® and all consecutive patients during the another time frame to muscle coverage. However, we have no reason to believe that there were any significant differences during the two time frames and the same surgeons operated the patients. Moreover, there were no demographic differences between the groups (Table 1), which supports that the two samples were similar. Secondly, muscle coverage is a method that has been used in our department for many years, whereas Surgisis® was a new technique and, therefore, there could have been a learning curve [21], potentially leading to more complications in the Surgisis® group. However, the results contradict that this was the case.
In conclusion, the complication rates in implant-based Surgisis® reconstruction seem to be similar to those seen with other matrices/meshes whereas expander-based Surgisis® reconstruction seems to result in a relatively high total early complication rate. Risk factors for early complications are mainly patient characteristics, such as a high BMI and smoking, as well as radiation, as with other matrices. The long-term capsular contracture rate and need for corrections, patient satisfaction and quality of life, are similar in the Surgisis® patients and muscle-covered controls.
References
Nguyen J, Carey J, Wong A (2011) Use of human acellular dermal matrix in implant-based breast reconstruction: evaluating the evidence. J Plast Reconstr Aesthet Surg 64:1553–1561
Spear SL, Parikh PM, Reisin E, Menon NG (2008) Acellular dermis-assisted breast reconstruction. Aesthet Plast Surg 32:418–425
Vardanian AJ, Clayton JL, Roostaeian J, Shirvanian V, Da Lio A, Lipa JE et al (2011) Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg 128:403e–410e
Breuing KH, Colwell AS (2007) Inferolateral AlloDerm hammock for implant coverage in breast reconstruction. Ann Plast Surg 59:250–255
Stump A, Holton LH 3rd, Connor J, Harper JR, Slezak S, Silverman RP (2009) The use of acellular dermal matrix to prevent capsule formation around implants in a primate model. Plast Reconstr Surg 124:82–91
Sbitany H, Serletti JM (2011) Acellular dermis-assisted prosthetic breast reconstruction: a systematic and critical review of efficacy and associated morbidity. Plast Reconstr Surg 128:1162–1169
Chun YS, Verma K, Rosen H, Lipsitz S, Morris D, Kenney P, Eriksson E (2010) Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg 125:429–436
de Blacam C, Momoh AO, Colakoglu S, Slavin SA, Tobias AM, Lee BT (2012) Cost analysis of implant-based breast reconstruction with acellular dermal matrix. Ann Plast Surg 69:516–520
Duncan DI (2001) Correction of implant rippling using allograft dermis. Aesthet Surg J 21:81–84
Breuing KH, Warren SM (2005) Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg 55:232–239
Hallberg H, Rafnsdottir S, Selvaggi G, Strandell A, Samuelsson O, Stadig I, Svanberg T, Hansson E, Lewin R (2018) Benefits and risks with acellular dermal matrix (ADM) and mesh support in immediate breast reconstruction: a systematic review and meta-analysis. J Plast Surg Hand Surg 52:130–147
Chan JC, Burugapalli K, Huang YS, Kelly JL, Pandit A (2017) A clinically relevant in vivo model for the assessment of scaffold efficacy in abdominal wall reconstruction. J Tissue Eng 8:2041731416686532
Rice RD, Ayubi FS, Shaub ZJ, Parker DM, Armstrong PJ, Tsai JW (2010) Comparison of Surgisis, AlloDerm, and Vicryl Woven Mesh grafts for abdominal wall defect repair in an animal model. Aesthet Plast Surg 34:290–296
Madani A, Niculiseanu P, Marini W, Kaneva PA, Mappin-Kasirer B, Vassiliou MC, Khwaja K, Fata P, Fried GM, Feldman LS (2017) Biologic mesh for repair of ventral hernias in contaminated fields: long-term clinical and patient-reported outcomes. Surg Endosc 31:861–871
Luo X, Kulig KM, Finkelstein EB, Nicholson MF, Liu XH, Goldman SM, Vacanti JP, Grottkau BE, Pomerantseva I, Sundback CA, Neville CM (2017) In vitro evaluation of decellularized ECM-derived surgical scaffold biomaterials. J Biomed Mater Res B Appl Biomater 105:585–593
Memon HU, El-Nashar SA, Thoreson AR, Weaver AL, Gebhart JB, Trabuco EC (2016) Comparison of graft-reinforced repairs and suture repair using a novel biomechanical test. Int Urogynecol J 27:47–53
Hunsicker LM, Ashikari AY, Berry C, Koch RM, Salzberg CA (2017) Short-term complications associated with acellular dermal matrix-assisted direct-to-implant breast reconstruction. Ann Plast Surg 78:35–40
Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ (2009) Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg 124:345–353
Cano SJ, Klassen AF, Scott AM, Cordeiro PG, Pusic AL (2012) The BREAST-Q: further validation in independent clinical samples. Plast Reconstr Surg 129:293–302
Sousa VD, Rojjanasrirat W (2011) Translation, adaptation and validation of instruments or scales for use in cross-cultural health care research: a clear and user-friendly guideline. J Eval Clin Pract 17:268–274
Lardi AM, Ho-Asjoe M, Mohanna PN, Farhadi J (2014) Immediate breast reconstruction with acellular dermal matrix: factors affecting outcome. J Plast Reconstr Aesthet Surg 67:1098–1105
Perez-Kohler B, Sotomayor S, Rodriguez M, Gegundez MI, Pascual G, Bellon JM (2015) Bacterial adhesion to biological versus polymer prosthetic materials used in abdominal wall defect repair: do these meshes show any differences in vitro? Hernia 19:965–973
Park TH, Chung SW, Song SY, Lew DH, Roh TS, Lee DW (2018) The use of acellular dermal matrix in immediate two-stage prosthetic breast reconstruction provides protection from postmastectomy radiation therapy: a clinicopathologic perspective. J Mater Sci Mater Med 29:27
Potter S, Browning D, Savovic J, Holcombe C, Blazeby JM (2015) Systematic review and critical appraisal of the impact of acellular dermal matrix use on the outcomes of implant-based breast reconstruction. Br J Surg 102:1010–1025
Martin L, O’Donoghue JM, Horgan K, Thrush S, Johnson R, Gandhi A, Association of Breast Surgery and the British Association of Plastic, Reconstructive and Aesthetic Surgeons (2013) Acellular dermal matrix (ADM) assisted breast reconstruction procedures: joint guidelines from the Association of Breast Surgery and the British Association of Plastic, Reconstructive and Aesthetic Surgeons. Eur J Surg Oncol 39:425–429
Wu C, Cipriano J, Osgood G Jr, Tepper D, Siddiqui A (2013) Human acellular dermal matrix (AlloDerm®) dimensional changes and stretching in tissue expander/implant breast reconstruction. J Plast Reconstr Aesthet Surg 66:1376–1381
Baldelli I, Cardoni G, Franchelli S, Fregatti P, Friedman D, Pesce M et al (2016) Implant-based breast reconstruction using a polyester mesh (Surgimesh-PET): a retrospective single-center study. Plast Reconstr Surg 137:931e–939e
Clarke-Pearson EM, Lin AM, Hertl C, Austen WG, Colwell AS (2016) Revisions in implant-based breast reconstruction: how does direct-to-implant measure up? Plast Reconstr Surg 137:1690–1699
Acknowledgements
We thank the statistician Aldina Pivodic, MSc, Statistika Konsultgruppen, who performed the statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was funded by the ALF Västra Götaland (agreement concerning research and education of doctors, Västra Götaland) and the Percy Falk Foundation for research into prostate cancer and breast cancer.
Conflict of interest
Håkan Hallberg, Richard Lewin, Madiha Bhatti Söftekand, Emmelie Widmark Jensen, Ulrika Kogler, Jonas Lundberg, and Emma Hansson declare that they have no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Ethical approval
This study was approved by the Regional Ethics Committee of Gothenburg (043-08).
Informed consent
Informed consent was obtained from all participants.
Additional information
Richard Lewin, Ulrika Kogler and Jonas Lundberg were affiliated at the time of data collection.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hallberg, H., Lewin, R., Søfteland, M.B. et al. Complications, long-term outcome and quality of life following Surgisis® and muscle-covered implants in immediate breast reconstruction: a case-control study with a 6-year follow-up. Eur J Plast Surg 42, 33–42 (2019). https://doi.org/10.1007/s00238-018-1444-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00238-018-1444-x