Abstract
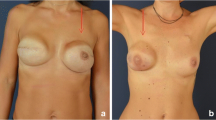
The submuscular implant-based breast reconstruction is the most common reconstructive technique following mastectomy. Recently, subcutaneous implant positioning, together with acellular dermal matrix, has become a promising technique in selected patients. We present the case of a 42-year-old woman who underwent left nipple-sparing mastectomy with prepectoral acellular dermal matrix (ADM) assisted direct-to-implant (DTI) breast reconstruction and contralateral mastopexy. The implant was completely wrapped around by Braxon®, a preshaped porcine ADM. A few months after surgery, she experienced a severe weight loss resulting in the aesthetic deterioration of both breasts. The patient showed a migration of the left implant inferiorly and laterally, and deflation of the contralateral breast. In order to improve the left breast, a lateral capsulectomy was performed to reduce the prepectoral pocket size and lift the implant. Subsequently, a modified donut mastopexy was performed to obtain an upward migration of the nipple-areolar complex. One of the limiting factors of prosthetic reconstruction, as compared to autologous reconstruction, is the aesthetic deterioration determined by any weight change. Differently from submuscular implant reconstruction, the prepectoral implant reconstruction follows body changes after weight changes and ageing. In fact, Braxon’s integration determines the formation of a capsule adhering to the mastectomy flap which makes the implant more sensible to dermatochalasis. The greater thickness of the mastectomy flap due to the larger representation of the subcutaneous tissue makes the reconstruction more sensitive to weight changes. A tailored partial capsulectomy combined with a donut mastopexy can be a solution in these patients after an important weight loss.
Level of Evidence: Level V, therapeutic study.




Similar content being viewed by others
References
American Society of Plastic Surgeons. 2014 Plastic Surgery Statistics Report. Available at: http://www.plasticsurgery.org/Documents/ news-resources/statistics/2014-statistics/plastic-surgery-statsitics- full-report.pdf. Accessed 25 Apr 2016
Sigalove S, Maxwell GP, Sigalove N, Storm-Dickerson TL, Pope N, Rice J, Gabriel A (2017) Prepectoral implant-based breast reconstruction: rationale, indications, and preliminary results. Plast Reconstr Surg 139(2):287–294
Snyderman RK, Guthrie RH (1971) Reconstruction of the female breast following radical mastectomy. Plast Reconstr Surg 47:565–567
Gruber RP, Kahn RA, Lash H, Maser MR, Apfelberg DB, Laub DR (1981) Breast reconstruction following mastectomy: a comparison of submuscular and subcutaneous techniques. Plast Reconstr Surg 67:312–317
Spear SL, Schwartz J, Dayan JH, Clemens MW (2009) Outcome assessment of breast distortion following submuscular breast augmentation. Aesthetic Plast Surg 33:44–48
Haynes DF, Kreithen JC (2014) Vicryl mesh in expander/implant breast reconstruction: long-term follow-up in 38 patients. Plast Reconstr Surg 134:892–899
Martin JB, Moore R, Paydar KZ, Wirth GA (2014) Use of fenestrations in acellular dermal allograft in two-stage tissue expander/implant breast reconstruction. Plast Reconstr Surg 134:901–904
Dieterich M, Angres J, Stubert J, Stachs A, Reimer T, Gerber B (2015) Patient-reported outcomes in implant-based breast reconstruction alone or in combination with a titanium-coated polypropylene mesh - a detailed analysis of the BREAST-Q and overview of the literature. Geburtshilfe Frauenheilkd 75:692–701
McCarthy CM, Lee CN, Halvorson EG, Riedel E, Pusic AL, Mehrara BJ et al (2012) The use of acellular dermal matrices in two-stage expander/implant reconstruction: a multicenter, blinded, randomized controlled trial. Plast Reconstr Surg 130(5 Suppl 2):57S–66S
Bernini M, Calabrese C, Cecconi L, Santi C, Gjondedaj U, Roselli J, Nori J, Fausto A, Orzalesi L, Casella D (2015) Subcutaneous direct-to- implant breast reconstruction: surgical, functional, and aesthetic results after long-term follow-up. Plast Reconstr Surg Glob Open 3:e574
Salibian AA, Frey JD, Choi M, Karp NS (2016) Subcutaneous implant-based breast reconstruction with acellular dermal matrix/mesh: a systematic review. Plast Reconstr Surg Glob Open 4(11):e1139
Zhu L, Mohan A, Abdelsattar JM, Wang Z, Vijayasekaran A, Michelle Hwang NV, Tran MD et al (2016) Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J Plast Reconstr Aesthet Surg 69(4):e77–e86
Casella D, Calabrese C, Bianchi S, eattini I, Bernini M (2015) Subcutaneous tissue expander placement with synthetic titanium-coated mesh in breast reconstruction: long-term results. Plast Reconstr Surg Glob Open 3:e577
Berna G, Cawthorn SJ, Papaccio G, Balestrieri N (2014) Evaluation of a novel breast reconstruction technique using the Braxon® acellular dermal matrix: a new muscle-sparing breast reconstruction. ANZ J Surg 87:493–498
Woods JE (1983) Subcutaneous mastectomy: current state of the art. Ann Plast Surg 11:541–550
Goodnight JE Jr, Quagliana JM, Morton DL (1984) Failure of subcutaneous mastectomy to prevent the development of breast cancer. J Surg Oncol 26:198–201
Reitsamer R, Peintinger F (2015) Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg 68:162–167
Wallace MS, Wallace AM, Lee J (1996) Pain after breast surgery: a survey of 282 women. Pain 66:195–205
Tomita K, Yano K, Nishibayashi A, Hosokawa K (2015) Effects of subcutaneous versus submuscular tissue expander placement on breast capsule formation. Plast Reconstr Surg Glob Open 3(6):e432
Becker H, Lind JG 2nd, Hopkins EG (2015) Immediate implant-based prepectoral breast reconstruction using a vertical incision. Plast Reconstr Surg Glob Open 3:e412
Arquero PS, Zanata FC, Ferreira LM, Nahas FX (2015) Capsular weakness around breast implant: a non-recognized complication. World J Plast Surg 4:168–74.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Patient consent
Written informed consent was obtained from the patient.
Ethical approval
For this type of study formal consent from a local ethics committee is not required.
Conflict of interest
Marzia Salgarello, Liliana Barone Adesi and Maria Lucia Mangialardi declare that they have no conflict of interest.
Funding
None.
Rights and permissions
About this article
Cite this article
Salgarello, M., Barone Adesi, L. & Mangialardi, M.L. A case of important weight loss after a prepectoral breast reconstruction. Eur J Plast Surg 41, 601–604 (2018). https://doi.org/10.1007/s00238-018-1413-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00238-018-1413-4