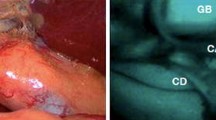
Abstract
Background
Near-infrared fluorescence (NIRF) imaging technique, after administration of contrast agents with fluorescent characteristics in the near-infrared (700–900 nm) range, is considered to possess great potential for the future of plastic surgery, given its capacity for perioperative, real-time anatomical guidance and identification. This study aimed to provide a comprehensive literature review concerning current and potential future applications of NIRF imaging in plastic surgery, thereby guiding future research.
Methods
A systematic literature search was performed in databases of Cochrane Library CENTRAL, MEDLINE, and EMBASE (last search Oct 2017) regarding NIRF imaging in plastic surgery. Identified articles were screened and checked for eligibility by two authors independently.
Results
Forty-eight selected studies included 1166 animal/human subjects in total. NIRF imaging was described for a variety of (pre)clinical applications in plastic surgery. Thirty-two articles used NIRF angiography, i.e., vascular imaging after intravenous dye administration. Ten articles reported on NIRF lymphography after subcutaneous dye administration. Although currently most applied, general protocols for dosage and timing of dye administration for NIRF angiography and lymphography are still lacking. Three articles applied NIRF to detect nerve injury, and another three studies described other novel applications in plastic surgery.
Conclusions
Future standard implementation of novel intraoperative optical techniques, such as NIRF imaging, could significantly contribute to perioperative anatomy guidance and facilitate critical decision-making in plastic surgical procedures. Further investigation (i.e., large multicenter randomized controlled trials) is mandatory to establish the true value of this innovative surgical imaging technique in standard clinical practice and to aid in forming consensus on protocols for general use.
Level of Evidence: Not ratable
Similar content being viewed by others
Introduction
Innovative optical imaging methods can be applied during surgery to detect and to differentiate tissues [1], a technique also known as image-guided surgery. A promising modality is near-infrared fluorescence (NIRF) imaging. After administration, contrast agents with fluorescent characteristics (i.e., fluorophores or fluorescent dyes) in the near-infrared range (NIR 700–900 nm) can be visualized using dedicated NIR camera systems. These fluorophores can be injected systemically (e.g., intravenously) or locally (e.g., subcutaneously). Indocyanine green (ICG) is the most common dye [2], but a variety of fluorophores can be applied. Currently, novel dyes with different chemical properties are being developed or tested in a preclinical setting in order to expand the potential of tissue differentiation, nerve detection in particular. Arteries, veins, ureters, lymph vessels, and lymph nodes have already successfully been identified using NIRF imaging in clinical trials [3,4,5]. A uniform approach regarding timing, dosage, and route of dye administration has not yet been established. The optimization of both imaging systems and fluorescent dyes is essential to improve current shortcomings [3].
A NIRF imaging system can be used by the surgeon in real time, thereby providing a significant advantage in terms of perioperative anatomical navigation and identification as well as facilitating the assessment of tissue perfusion or viability [1]. The NIRF imaging technique is currently being implemented in most new microscopic surgical systems. Since many plastic surgery departments possess a microscope, it will probably become easily accessible for the general field.
This review aims to provide a comprehensive insight into the current and potential future applications of NIRF imaging for perioperative anatomical guidance in the field of plastic and reconstructive surgery. Directions and implications for future research are given.
Methods
This study was conducted according to the PRISMA standard for systematic reviews (see Electronic Supplementary Material for PRISMA Checklist) [6]. A systematic literature search was performed in October 2017 in the following databases: Cochrane Library database CENTRAL, MEDLINE, and EMBASE. Both structured MeSH terms and free terms were used in the PubMed search. The terms applied were such that any description that could resemble or relate to the use of NIRF imaging in plastic and reconstructive surgery would be uncovered by the search; Table 1 displays an overview of the search terms. Additional literature was collected after scanning the reference lists of existing review articles.
Two investigators (R.S. and A.C.) independently performed the literature selection. A third investigator (X.K.) was available for consultation in case of disagreement. Inclusion of an article resulted from a three-phase process that consisted of the initial literature search, screening of the literature resulting from the search, and evaluation of eligibility of the articles provided by the screening. Neither language nor publication date or publication status restrictions were applied. Both clinical and preclinical studies were included; systematic reviews and meta-analysis were excluded. A substantive evaluation of NIRF systems and their corresponding NIRF imaging performance is not within the scope of this review.
Eligibility of the studies was based on the following criteria:
-
Does the study report on NIRF imaging in plastic and reconstructive surgery?
-
Does the paper describe an application of NIRF imaging for enhanced anatomical guidance or assessment of tissue perfusion?
-
Does the article provide insight into future applications of NIRF image-guided plastic surgery?
Primarily, titles and abstracts were screened. In case of incertitude, full-text reports were read to determine eligibility. Reference lists of the selected articles were also screened based on the previously described criteria. A data extraction sheet was developed containing items on the aim of the study, the imaging system that was used, and the fluorescent dye and administration. The data extraction sheet was completed for all eligible studies by three independent authors (A.C., R.S., and C.G.).
Results
Following the systematic literature search, a total of 94 studies were identified. After reviewing the title and abstract, 44 hits were directly excluded. Another two were excluded after reading the full article. The main reason for exclusion: NIRF imaging was used in another surgical specialty than plastic and reconstructive surgery (n = 38, e.g., general surgery, neurosurgery, urology, or dermatology). Other reasons for exclusion: NIRF was used in a molecular study, a review was presented (n = 4), or the cost-effectiveness of the device itself was explored (n = 2). A detailed overview of the study selection is presented in Fig. 1.
Ultimately, 48 studies were eligible within the scope of this review (covering a total of 1166 animal/human subjects). The selected studies—all written in the English language—were published within the period from 2007 until 2017. Fifteen articles reported on animal experiments, and the remainder described clinical findings. The content of the selected articles will be presented following three subcategories, respectively: NIRF imaging systems (see Table 2), NIR fluorescent dyes (see Table 3), and applications of NIRF imaging in plastic and reconstructive surgery (see Table 4, 5, 6, and 7).
NIRF imaging systems
Various NIRF imaging systems have been described in the literature, as summarized in Table 2. In the described experiments, hand-held imaging systems and microscopes with an integrated NIRF were equally divided for imaging. There were four different hand-held systems (PDE n = 13, Visionsense n = 1, Fluobeam n = 2, and HyperEye n = 1) [7,8,9,10,11,12,13,14,15,16,17,18,19, 44,45,46, 49], one non-hand-held system (FLARE n = 8) [36,37,38,39,40,41,42,43], and three types of microscopes with an integrated NIRF (SPY n = 16, LEICA n = 2, and Pentero n = 1) [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35, 47, 48] suitable for fluorescence image guidance. In one study, a prototype was used which was not further specified [51]; three articles unfortunately did not state what kind of imaging system was used [52,53,54].
NIR fluorescent dyes
A handful of NIR fluorophores are reported in the literature (see Table 3). Indocyanine green (ICG) and methylene blue (MB) are two clinical fluorophores. Preclinical dyes have also been under investigation. Currently, the maximum penetration depth of NIRF visualization of ICG or MB is limited to 1.0–1.5 cm. The use of ICG was described in 44 articles [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39, 41,42,43,44,45,46,47,48,49,50,51], thereby making it by far the most commonly administered dye. ICG was injected either subcutaneously in order to visualize superficial lymphatic vessels (i.e. lymphography) or intravenously in order to assess flap, composite allograft, or bone perfusion (i.e., angiography).
One study used intravenous MB to assess flap perfusion [40]. Although only once reported in plastic surgery literature, methylene blue is in fact a potential dye for near-infrared fluorescence imaging at around 700 nm.
Five different preclinical dyes were tested in animal studies in order to detect nerve injury by intraneural injection of the dye [53, 54]. One study labeled fat cells with a specific fluorescent dye to enable the investigation of the amount of fat cells, which survived after autologous fat cell transportation [52].
No side effects due to the administered dye were reported in the included studies. Nevertheless, although rare, the reported rates of severe and moderate reactions to ICG are approximately 0.07–0.1%. Additionally, methylene blue is also known to potentially cause severe allergic reactions as well.
Applications of NIRF imaging in plastic and reconstructive surgery
NIRF imaging has already been explored for multiple applications in plastic surgery, either in an animal study or in a clinical setting. An overview of applications for tissue navigation is displayed in Table 4, 5, 6, and 7. Undoubtedly, angiography and lymphography are currently the two most used NIRF applications in plastic surgery.
NIRF angiography, after intravenous dye administration, was reported in 32 articles. The majority (n = 24) [7, 8, 10, 11, 15, 20,21,22, 24, 27, 29, 32, 35, 36, 40,41,42,43,44, 48,49,50,51] used NIRF to assess tissue perfusion in (free) flap surgery; the remainder focused on the perioperative assessment of mastectomy skin flap perfusion [32,33,34], bone perfusion [37], abdominal wall perfusion in abdominal wall reconstruction [23, 28], and perfusion of a composite allograft [25, 38, 39]. See Table 4. When reported, intravenous ICG dosage for perfusion imaging ranged from 0.025 to 0.50 mg/kg.
Ten articles [9, 12,13,14, 16, 19, 30, 31, 46, 47] used NIRF lymphography after subcutaneous/intradermal administration for a variety of reasons: to plan a lymphaticovenous anastomosis (LVA), to stage lymphedema, or to assess lymphatic flow in a composite allograft (e.g., vascularized lymph node transplants). See Table 5. When reported, the ICG dosage for lymphography ranged from 0.03 to 0.25 mg, which was administered subcutaneously/intradermally.
Three articles [18, 53, 54] injected a preclinical dye intraneurally to check for nerve injury (see Table 6).
There are some other novel applications within the field of plastic surgery (see Table 7). Dye administration in autologous transplanted fat tissue, for example, was investigated to assess the amount of fat cells that survived [52]. Another article [26] used NIRF to assess perfusion after revascularization of upper limb extremity ischemia. The application of NIRF imaging to determine tissue necrosis in open lower-limb fractures was also reported [17].
Unfortunately, the dosage and timing of administration of the different types of dye for the variety of aforementioned applications is either poorly documented and/or no consensus is available. A worldwide-accepted protocol for general clinical use is lacking. This would be of particular interest for the already clinically available dyes and applications.
Discussion
The aim of this review was to evaluate the current applications (including available imaging systems and fluorescent dyes) and potential future applications of NIRF imaging in plastic and reconstructive surgery. NIRF imaging has shown potential for identification of several vital anatomical structures (e.g., arteries, veins, lymph vessels), even when covered under a layer of adipose or connective tissue. NIRF imaging can visualize vessels up to 1.5 cm subcutaneously [55]. These are all hollow structures that can be delineated using endo-luminal transported agents. Nerves have also been illuminated by intraneural injection and a dye, which is hydrophilic. However, future fluorescent dyes have been reported that will allow for solid anatomical structures to be visualized through NIRF imaging using specific peptides as targets [53, 56, 57]. The latter underlines the value for including animal studies within the current review. Preclinical fluorescent dyes have to be evaluated in animal setting first prior to human testing and validation. Inclusion of both animal and clinical studies is valuable to forecast future perspectives.
The current study comprises the first review in which all aspects (imaging systems, dyes, and clinical applications) of NIRF imaging within plastic and reconstructive surgery is discussed. Previous reviews only focused on one specific type of dye (ICG) or mainly one type of application. For example, Burnier et al. published a review on ICG applications in plastic surgery. Approximately half of their included studies reports on guidance during sentinel lymph node biopsy [58]. Liu et al. published a review on perioperative ICG angiography [59]. In both reviews, only ICG is used as NIRF dye.
From the systematic literature search, it can be concluded that NIRF is mainly used for angiography (e.g., flap perfusion) and lymphography (e.g., for perioperative planning of LVA and staging of lymphedema). Only a minority has described the potential for neurography using NIRF. However, in plastic and reconstructive surgery, enhanced nerve detection would also be of particular interest, for example in detecting or excluding nerve injury (i.e., differentiating between nerve injury versus neuropraxia), in the treatment of traumatic amputation of digit(s), guiding sensory free flap surgery, or facial nerve surgery.
Besides the aforementioned studies, publications on novel applications of NIRF image guidance in plastic surgery are scarce. Bliley et al. describe an in vivo technique in which stromal vascular fraction within autologous fat grafts can be tracked by NIRF [52]. This technique offers potential to determine the prevalence and destiny of injected fat cells in the future, thereby giving it a role in autologous fat grafts in reconstructive surgery, a surgical procedure which is being increasingly implemented in daily clinical practice and may become the future for the reconstruction of defects.
In case of a trauma, NIRF could be a convenient tool to determine soft tissue injury and necrosis thereby guiding trauma debridement. Koshimune et al. used NIRF to designate necrosis and reduce the number of debridement after open lower-limb fractures [17]. A precise assessment of skin defect size and the presence or absence of necrotic tissue can be useful in an estimation of flap size. Brooks et al. used NIRF to assess perfusion after revascularization of upper limb extremity ischemia. NIRF was used to increase understanding of the physiology of arterial-venous reversal in patients with terminal ischemia of an upper limb [26].
At the moment, ICG is the most frequently used dye in NIRF in plastic surgery. One of the advantages of ICG for NIRF angiography in particular is the quick half-life of 3–4 min in healthy adults. Therefore, it can be used several times for imaging without exceeding the maximal dosage [60]. In the available literature, dosage of different types of dye is either poorly documented or no consensus is present. Time between injecting and NIRF imaging, as well as distance of the camera to the target-tissue, is not unanimously defined. Currently, there is no standard protocol on dosage and timing of dye administration for general use.
Furthermore, no consensus is available on subcutaneous injection of ICG to visualize lymphatic vessels. In perioperative planning of LVA and staging lymphedema, ICG is injected subcutaneously in one or more of the web spaces of the foot or the hand depending on the location of the lymphedema. Additional injections are also given subcutaneously at the medial and volar side of the hand or at the medial and lateral side of the Achilles tendon. No agreement has yet been reached about which web space should be used, and whether additional injections are in fact necessary.
This review presented some limitations. The level of evidence of the included studies is rather low. Only one randomized clinical trial on abdominal wall perfusion could be included [23]. The majority of the studies were case reports, cohort studies, or (pre)clinical feasibility studies without a clear protocol regarding dosage, time of imaging, and administration. From this regard, a meta-analysis of available data could not be performed. Nevertheless, this study gives a comprehensive overview of the use of NIRF in the field of plastic surgery.
Further trials are needed to establish consensus regarding standard protocols for angiography and lymphography, two applications which are currently most applied within plastic and reconstructive surgery. This could be achieved by conducting (large, multicenter) randomized controlled trials. Next, other NIRF applications within plastic surgery need to be explored more extensively, such as NIRF-guided trauma debridement. Moreover, the imaging technique itself needs to be improved: more potent and powerful dyes would increase the range of applications as well as the penetration depth in tissues.
Conclusion
Future standard implementation of novel intraoperative optical techniques, such as NIRF imaging, could significantly contribute to perioperative anatomy guidance and facilitate critical decision-making in plastic surgical procedures. Further investigation (i.e., large multicenter randomized controlled trials) is mandatory to establish the true value of this innovative surgical imaging technique in standard clinical practice and to aid in forming consensus on protocols for general use.
References
Schols RM, Bouvy ND, van Dam RM, Stassen LP (2013) Advanced intraoperative imaging methods for laparoscopic anatomy navigation: an overview. Surg Endosc 27:1851–1859
Schaafsma BE, Mieog JS, Hutteman M et al (2011) The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol 104:323–332
Schols RM, Connell NJ, Stassen LP (2015) Near-infrared fluorescence imaging for real-time intraoperative anatomical guidance in minimally invasive surgery: a systematic review of the literature. World J Surg 39:1069–1079
Schaafsma BE, Verbeek FP, Rietbergen DD et al (2013) Clinical trial of combined radio- and fluorescence-guided sentinel lymph node biopsy in breast cancer. Br J Surg 100:1037–1044
Al-Taher M, van den Bos J, Schols RM, Bouvy ND, Stassen LP (2016) Fluorescence ureteral visualization in human laparoscopic colorectal surgery using methylene blue. J Laparoendosc Adv Surg Tech A 26:870–875
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62:1006–1012
Xu H, Zhang Z, Xia Y, Steinberger Z, Min P, Li H, Dai Y, Zhang Y (2016) Preliminary exploration: when angiosome meets prefabricated flaps. J Reconstr Microsurg 32:683–687
Xu H, Zhang Z, Tao W, Steinberger Z, Li H, Levin L, Fang Y, Zhang Y (2016) Ex vivo delay: a novel approach to increase prefabricated flaps survival rate. J Reconstr Microsurg 32:632–638
Narushima M, Yamamoto T, Ogata F, Yoshimatsu H, Mihara M, Koshima IIG (2016) Lymphography findings in limb lymphedema. J Reconstr Microsurg 32:72–79
Hayashi A, Yoshizawa H, Tanaka R, Natori Y, Arakawa A, Mizuno H (2015) Intraoperative use of Indocyanine green fluorescence angiography during distally based radial artery perforator flap for squamous cell carcinoma of the thumb. Plast Reconstr Surg Glob Open 3:e310
Nagata T, Masumoto K, Uchiyama Y, Watanabe Y, Azuma R, Morimoto Y, Katou F (2014) Improved technique for evaluating oral free flaps by pinprick testing assisted by indocyanine green near-infrared fluorescence angiography. J Craniomaxillofac Surg 42:1112–1116
Yamamoto T, Narushima M, Yoshimatsu H, Yamamoto N, Kikuchi K, Todokoro T, Iida T, Koshima I (2014) Dynamic Indocyanine green (ICG) lymphography for breast cancer-related arm lymphedema. Ann Plast Surg 73:706–709
Mundinger GS, Narushima M, Hui-Chou HG, Jones LS, Ha JS, Shipley ST, Drachenberg CB, Dorafshar AH, Koshima I, Bartlett ST, Barth RN, Rodriguez ED (2012) Infrared fluorescence imaging of lymphatic regeneration in nonhuman primate facial vascularized composite allografts. Ann Plast Surg 68:314–319
Maegawa J, Yabuki Y, Tomoeda H, Hosono M, Yasumura K (2012) Outcomes of lymphaticovenous side-to-end anastomosis in peripheral lymphedema. J Vasc Surg 55:753–760
Quilichini J, Le Masurier P, Guihard T (2010) Increasing the reliability of SIEA flap using peroperative fluorescent angiography with indocyanine green in breast reconstruction. Ann Chir Plast Esthet 55:531–538
Ogata F, Narushima M, Mihara M, Azuma R, Morimoto Y, Koshima I (2007) Intraoperative lymphography using indocyanine green dye for near-infrared fluorescence labeling in lymphedema. Ann Plast Surg 59:180–184
Koshimune S, Shinaoka A, Ota T, Onoda S, Kimata YL-AI (2017) Green angiography aids in the reconstruction of Gustilo grade IIIB open lower-limb fractures. J Reconstr Microsurg 33:143–150
Tanaka K, Okazaki M, Yano T, Miyashita H, Homma T, Tomita MQ (2015) Evaluation of blood perfusion to nerves included in the anterolateral thigh flap using indocyanine green fluorescence angiography: a different contrast pattern between the vastus lateralis motor nerve and femoral cutaneous nerve. J Reconstr Microsurg 31:163–170
Chang DW, Suami H, Skoracki R (2013) A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg 132:1305–1314
Daram SP, Sacks JM, Kupferman ME (2015) Noninvasive intraoperative angiography for reconstruction of head and neck defects. Ear Nose Throat J 94:E32–E36
Ludolph I, Arkudas A, Schmitz M, Boos AM, Taeger CD, Rother U, Horch RE, Beier JP (2016) Cracking the perfusion code?: laser-assisted Indocyanine green angiography and combined laser Doppler spectrophotometry for intraoperative evaluation of tissue perfusion in autologous breast reconstruction with DIEP or ms-TRAM flaps. J Plast Reconstr Aesthet Surg 69:1382–1388
Diep GK, Hui JY, Marmor S et al (2016) Postmastectomy reconstruction outcomes after intraoperative evaluation with Indocyanine green angiography versus clinical assessment. Ann Surg Oncol 23:4080–4085
Wormer BA, Huntington CR, Ross SW, Colavita PD, Lincourt AE, Prasad T, Sing RF, Getz SB, Belyansky I, Heniford BT, Augenstein VA (2016) A prospective randomized double-blinded controlled trial evaluating indocyanine green fluorescence angiography on reducing wound complications in complex abdominal wall reconstruction. J Surg Res 202:461–472
Nasser A, Fourman MS, Gersch RP et al (2015) Utilizing indocyanine green dye angiography to detect simulated flap venous congestion in a novel experimental rat model. J Reconstr Microsurg 31:590–596
Valerio I, Green JM 3rd, Sacks JM, Thomas S, Sabino J, Acarturk TO (2015) Vascularized osseous flaps and assessing their bipartate perfusion pattern via intraoperative fluorescence angiography. J Reconstr Microsurg 31:45–53
Brooks D (2014) Perfusion assessment with the SPY system after arterial venous reversal for upper extremity ischemia. Plast Reconstr Surg Glob Open 2:e185
Munabi NC, Olorunnipa OB, Goltsman D, Rohde CH, Ascherman JA (2014) The ability of intra-operative perfusion mapping with laser-assisted indocyanine green angiography to predict mastectomy flap necrosis in breast reconstruction: a prospective trial. J Plast Reconstr Aesthet Surg 67:449–455
Patel KM, Bhanot P, Franklin B, Albino F, Nahabedian MY (2013) Use of intraoperative indocyanin-green angiography to minimize wound healing complications in abdominal wall reconstruction. J Plast Surg Hand Surg 47:476–480
Wu C, Kim S, Halvorson EG (2013) Laser-assisted indocyanine green angiography: a critical appraisal. Ann Plast Surg 70:613–619
Shih HB, Shakir A, Nguyen DH (2016) Use of indocyanine green-SPY angiography for tracking lymphatic recovery after lymphaticovenous anastomosis. Ann Plast Surg 76(Suppl 3):S232–S237
Chen WF, Zhao H, Yamamoto T, Hara H, Ding J (2016) Indocyanine green lymphographic evidence of surgical efficacy following microsurgical and Supermicrosurgical lymphedema reconstructions. J Reconstr Microsurg 32:688–698
Komorowska-Timek E, Gurtner GC (2010) Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg 125:1065–1073
Hammer-Hansen N, Juhl AA, Damsgaard TE (2017) Laser-assisted indocyanine green angiography in implant-based immediate breast reconstruction: a retrospective study. J Plast Surg Hand Surg :1–5
Bertoni DM, Nguyen D, Rochlin D, Hernandez-Boussard T, Meyer S, Choy N, Gurtner GC, Wapnir IL (2016) Protecting nipple perfusion by devascularization and surgical delay in patients at risk for ischemic complications during nipple-sparing mastectomies. Ann Surg Oncol 23:2665–2672
Newman MI, Samson MC (2009) The application of laser-assisted indocyanine green fluorescent dye angiography in microsurgical breast reconstruction. J Reconstr Microsurg 25:21–26
Vargas CR, Nguyen JT, Ashitate Y, Silvestre J, Venugopal V, Neacsu F, Kettenring F, Frangioni JV, Gioux S, Lee BT (2015) Near-infrared imaging for the assessment of anastomotic patency, thrombosis, and reperfusion in microsurgery: a pilot study in a porcine model. Microsurgery 35:309–314
Nguyen JT, Ashitate Y, Buchanan IA, Ibrahim AMS, Gioux S, Patel PP, Frangioni JV, Lee BT (2012) Bone flap perfusion assessment using near-infrared fluorescence imaging. J Surg Res 178:e43–e50
Nguyen JT, Ashitate Y, Buchanan IA, Ibrahim AMS, Gioux S, Patel PP, Frangioni JV, Lee BT (2012) Face transplant perfusion assessment using near-infrared fluorescence imaging. J Surg Res 177:e83–e88
Nguyen JT, Ashitate Y, Venugopal V, Neacsu F, Kettenring F, Frangioni JV, Gioux S, Lee BT (2013) Near-infrared imaging of face transplants: are both pedicles necessary? J Surg Res 184:714–721
Ashitate Y, Lee BT, Laurence RG, Lunsford E, Hutteman M, Oketokoun R, Choi HS, Frangioni JV (2013) Intraoperative prediction of postoperative flap outcome using the near-infrared fluorophore methylene blue. Ann Plast Surg 70:360–365
Matsui A, Lee BT, Winer JH, Kianzad V, Frangioni JV (2009) Image-guided perforator flap design using invisible near-infrared light and validation with x-ray angiography. Ann Plast Surg 63:327–330
Matsui A, Lee BT, Winer JH, Laurence RG, Frangioni JV (2010) Predictive capability of near-infrared fluorescence angiography in submental perforator flap survival. Plast Reconstr Surg 126:1518–1527
Lee BT, Hutteman M, Gioux S, Stockdale A, Lin SJ, Ngo LH, Frangioni JV (2010) The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in perforator flap breast reconstruction. Plast Reconstr Surg 126:1472–1481
Bigdeli AK, Gazyakan E, Schmidt VJ, Hernekamp FJ, Harhaus L, Henzler T, Kremer T, Kneser U, Hirche C (2016) Indocyanine green fluorescence for free-flap perfusion imaging revisited: advanced decision making by virtual perfusion reality in Visionsense fusion imaging angiography. Surg Innov 23:249–260
Hitier M, Cracowski JL, Hamou C, Righini C, Bettega G (2016) Indocyanine green fluorescence angiography for free flap monitoring: a pilot study. J Craniomaxillofac Surg 44:1833–1841
Miranda Garces M, Pons G, Mirapeix R, Masia J (2017) Intratissue lymphovenous communications in the mechanism of action of vascularized lymph node transfer. J Surg Oncol 115:27–31
Liu HL, Pang SY, Chan YW (2014) The use of a microscope with near-infrared imaging function in indocyanine green lymphography and lymphaticovenous anastomosis. J Plast Reconstr Aesthet Surg 67:231–236
Sugawara J, Satake T, Muto M, Kou S, Yasumura K, Maegawa J (2015) Dynamic blood flow to the retrograde limb of the internal mammary vein in breast reconstruction with free flap. Microsurgery 35:622–626
Kuriyama M, Yano A, Yoshida Y, Kubo M, Akita S, Mitsukawa N, Satoh K, Yamamoto S, Sasaguri S, Orihashi K (2016) Reconstruction using a divided latissimus dorsi muscle flap after conventional posterolateral thoracotomy and the effectiveness of indocyanine green-fluorescence angiography to assess intraoperative blood flow. Surg Today 46:326–334
Holm C, Mayr M, Hofter E, Dornseifer U, Ninkovic M (2009) Assessment of the patency of microvascular anastomoses using microscope-integrated near-infrared angiography: a preliminary study. Microsurgery 29:509–514
Watson JR, Gainer CF, Martirosyan N, Skoch J, Lemole GM Jr, Anton R, Romanowski M (2015) Augmented microscopy: real-time overlay of bright-field and near-infrared fluorescence images. J Biomed Opt 20:106002
Bliley JM, Satish L, McLaughlin MM, Kling RE, Day JR, Grahovac TL, Kokai LE, Zhang W, Marra KG, Rubin JP (2015) Imaging the stromal vascular fraction during soft-tissue reconstruction. Plast Reconstr Surg 136:1205–1215
Gustafson TP, Yan Y, Newton P, Hunter DA, Achilefu S, Akers WJ, Mackinnon SE, Johnson PJ, Berezin MY (2012) A NIR dye for development of peripheral nerve targeted probes. Medchemcomm 3:685–690
Zhou H, Yan Y, Ee X, Hunter DA, Akers WJ, Wood MD, Berezin MY (2016) Imaging of radicals following injury or acute stress in peripheral nerves with activatable fluorescent probes. Free Radic Biol Med 101:85–92
Azuma R, Morimoto Y, Masumoto K, Nambu M, Takikawa M, Yanagibayashi S, Yamamoto N, Kikuchi M, Kiyosawa T (2008) Detection of skin perforators by indocyanine green fluorescence nearly infrared angiography. Plast Reconstr Surg 122:1062–1067
Gibbs-Strauss SL, Nasr KA, Fish KM, Khullar O, Ashitate Y, Siclovan TM, Johnson BF, Barnhardt NE, Tan Hehir CA, Frangioni JV (2011) Nerve-highlighting fluorescent contrast agents for image-guided surgery. Mol Imaging 10:91–101
Whitney MA, Crisp JL, Nguyen LT, Friedman B, Gross LA, Steinbach P, Tsien RY, Nguyen QT (2011) Fluorescent peptides highlight peripheral nerves during surgery in mice. Nat Biotechnol 29:352–356
Burnier P, Niddam J, Bosc R, Hersant B, Meningaud JP (2017) Indocyanine green applications in plastic surgery: a review of the literature. J Plast Reconstr Aesthet Surg 70:814–827
Liu DZ, Mathes DW, Zenn MR, Neligan PC (2011) The application of indocyanine green fluorescence angiography in plastic surgery. J Reconstr Microsurg 27:355–364
Meijer DK, Weert B, Vermeer GA (1988) Pharmacokinetics of biliary excretion in man. VI. Indocyanine green. Eur J Clin Pharmacol 35:295–303
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Anouk J.M. Cornelissen, Tom J.M. van Mulken, Caitlin Graupner, Shan S. Qiu, Xavier H.A. Keuter, René R.W.J. van der Hulst and Rutger M. Schols declare that they have no conflict of interest.
Funding
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Ethical approval
Not applicable for this article type.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Cornelissen, A.J.M., van Mulken, T.J.M., Graupner, C. et al. Near-infrared fluorescence image-guidance in plastic surgery: A systematic review. Eur J Plast Surg 41, 269–278 (2018). https://doi.org/10.1007/s00238-018-1404-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00238-018-1404-5