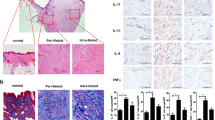
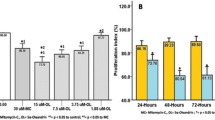
Abstract
Wound healing can lead to hypertrophic scar or keloid formation, characterized by an overabundant extracellular matrix. Current established treatment strategies include surgical resection, triamcinolone steroid injection, pressure therapy, silicone therapy, radiotherapy, etc. Cytokines also play a critical role in the regulation of cellular activities and extracellular matrix metabolism. Interferons (IFN) represent a group of antifibroproliferative agents that inhibit fibroblast proliferation and collagen production, and interleukin (IL)-1β also accelerates hypertrophic scar fibroblasts to produce collagenolytic enzymes, leading to tissue destruction. This study addressed the effects of steroid, IFN α-2b, or IL-1β on apoptosis and cell pathway of fibroblasts from keloids, hypertrophic scars, and normal skins and different responses of different fibroblasts. Six samples of keloid, six samples of hypertrophic scar, and six samples of normal skin were, respectively, collected from patients, and fibroblasts from different sources were cultured in vitro. After different fibroblasts were treated with dexamethasone (0.1 mg/ml) or IFN α-2b (1,000 μ/ml) or IL-1β (200 μ/ml), Bax and Bcl-2 were detected in situ by immunohistochemical staining; deoxyribonucleic acid ladders of different fibroblasts were observed by gel electrophoresis, and relative activated (phospho-) extracellular-signal-regulated kinase (ERK) 1/2 and c-Jun N-terminal kinase (JNK) pathways were detected by the method of fast activated cell-based enzyme-linked immunosorbent assay. In media containing dexamethasone, apoptosis took place in fibroblasts from keloids, hypertrophic scars, and normal skins by gel electrophoresis with increased rate of Bax/Bcl-2. Activated (phospho-) ERK1/2 and activated (phospho-) JNK expressions increased in three different fibroblasts. In media containing IFN α-2b, no apoptosis took place in three different fibroblasts without any change of expressions of Bax and Bcl-2 except for the expression of decreased Bcl-2 in fibroblasts from keloids. Activated (phospho-) ERK1/2 expression decreased in fibroblasts from keloid and hypertrophic scars without any changes of activated (phospho-) JNK expression, and IFN α-2b did not affect both activated (phospho-) ERK1/2 and activated (phospho-) JNK expressions in fibroblasts from normal skin. In media containing IL-1β, apoptosis of fibroblasts from keloids was induced by stimulating activated (phospho-) ERK1/2 and activated (phospho-) JNK pathways; IL-1β could not induce apoptosis of fibroblasts from normal skin (radio of Bax/Bcl-2 decreasing) whose activated (phospho-) ERK1/2 pathway was stimulated without any changes of activated (phospho-) JNK expression. Apoptosis in fibroblasts from hypertrophic scars was induced by activating the JNK pathway and prohibiting the ERK1/2 pathway. The effects of steroid, IFN α-2b, or IL-1β on apoptosis of different fibroblasts were different through different cell signal pathways, although all of them were effective for treatment of abnormal scars.











Similar content being viewed by others
References
Duncan MR, Berman B (1989) Differential regulation of collagen, glycosaminoglycan, fibronectin, and collagenase activity production in cultured human adult dermal fibroblasts by interleukin 1-alpha and beta and tumor necrosis factor-alpha and beta. J Invest Dermatol 92:699–706
Yoshizaki K, Nishimoto N, Kishimoto T (2002) IL-6 blocking therapy with humanized anti-IL-6 receptor antibody (MRA) in rheumatoid arthritis. Cutting Edge Reports. Available at: http://www.rheuma21st.com (August)
Kovacs EJ (1991) Fibrogenic cytokines: the role of immune mediators in the development of scar tissue. Immunol Today 12:17–23
Dasu MR, Hawkins HK, Barrow RE et al (2004) Gene expression profiles from hypertrophic scar fibroblasts before and after IL-6 stimulation. J Pathol 202:476
Luo S, Benathan M, Raffoul W et al (2001) Abnormal balance between proliferation and apoptotic cell death in fibroblasts derived from keloid lesions. Plast Reconstr Surg 107:87–96
Appleton I, Brown NJ, Willoughby DA (1996) Apoptosis, necrosis, and proliferation: Possible implications in the etiology of keloids. Am J Pathol 149:1441–1447
Desmouliere A, Redard M, Darby I et al (1995) Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 146:56–66
Sayah DN, Soo C, Shaw WW et al (1999) Downregulation of apoptosis-related genes in keloid tissues. J Surg Res 87:209–216
Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305:626–629
Cory S, Huang DC, Adams JM (2003) The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene 22:8590–8607
Kirkin V, Joos S, Zornig M (2004) The role of Bcl-2 family members in tumorigenesis. Biochim Biophys Acta 1644:229–249
Yang SH, Chien CM, Lu MC et al (2006) Up-regulation of Bax and endonuclease G, and down-modulation of Bcl-XL involved in cardiotoxin III-induced apoptosis in K562 cells. Exp Mol Med 38:435–444
Adams JM, Harris AW, Strasser A et al (1999) Transgenic models of lymphoid neoplasia and development of a panhematopoietic vector. Oncogene 18:5268–5277
Oltvai ZN, Milliman CL, Lorsmeger SJ (1993) Bcl-2heterdimerizes in vivo with a conserved homology, Bax, that accelerates programmed cell death. Cell 74:609–621
Ballif BA, Blenis J (2001) Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)–MAPK cell survival signals. Cell Growth Differ 12:397–408
Pearson G, Robinson F, Beers Gibson T et al (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22:153–183
Finlay GA, Thannickal VJ, Fanburg BL et al (2000) Transforming growth factor-. b. 1-induced activation of the ERK pathway/activator protein-1 in human lung fibroblasts requires the autocrine induction of basic fibroblast growth factor. J Biol Chem 275:27650–27656
Xia Z, Dickens M, Raingeaud J et al (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326–1331
Fettucciari K, Fetriconi I, Bartoli A et al (2003) Involvement of mitogen-activated protein kinases in Group B Streptococcus-induced macrophage apoptosis. Pharmacol Res 47:355–362
Daian T, Ohtsuru A, Rogounovitch T et al (2003) Insulinlike growth factor-I enhances transforming growth factor-induced extracellular matrix protein production through the p38/activating transcription factor-2 signaling pathway in keloid fibroblasts. J Invest Dermatol 120:956–962
Gupta K, Kshirsagar S, Li W et al (1999) VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res 247:495–504
Widmann C, Gibson S, Jarpe MB et al (1999) Mitogen-activated protein kinase. Conservation of a three-kinase module from yeast to human. Physiol Rev 79:143–180
Cobb MH, Boulton TG, Robbins DJ (1991) Extracellular signal-regulated kinase: ERKs in progress. Cell Regul 2:965–978
Thomas G (1992) MAPkinase by any other name smells just as sweet. Cell 68:3–6
Burgering BM, de Vries-Smits AM, Medema RH et al (1993) Epidermal growth factor induces phosphorylation of extracellular signal-regulated kinase 2 via multiple pathways. Mol Cell Biol 13:7248–7256
Szabowski A, Maas-Szabowski N, Andrecht S et al (2000) c-Jun and JunB antagonistically control cytokine-regulated mesenchymal–epidemal interaction in skin. Cell 103:745–755
Liu ZG, Hsu H, Goeddel DV et al (1996) Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell 87:565–576
Yujiri T, Sather S, Fanger GR et al (1998) Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science 282:1911–1914
Kauh YC, Rouda S, Mondragon G et al (1997) Major suppression of pro-alpha1(I) type I collagen gene expression in the dermis after keloid excision and immediate intrawound injection of triamcinolone acetonide. J Am Acad Dermatol 37:586–589
Poochareon VN, Berman B (2003) New therapies for the management of keloids. J Craniofacial Surg 14:654–657
Harrop AR, Ghahary A, Scott PG et al (1995) Regulation of collagen synthesis and mRNA expression in normal and hypertrophic scar fibroblasts in vitro by interferon-g. J Surg Res 58:471–477
Duncan MR, Berman B (1989) Differential regulation of glycosaminoglycan, fibronectin, and collagenase production in cultured human dermal fibroblasts by interferon-α, -β, and -γ. Arch Dermatol Res 281:11–18
Tamai K, Ishikawa H, Mauviel A et al (1995) Interferon-g coordinately upregulates matrix metalloproteinase (MMP)-1 and MMP-3, but not tissue inhibitor of metalloproteinases (TIMP), expression in cultured keratinocytes. J Invest Dermatol 104:384–390
Gurujey Alakshmi G, Giri SN (1995) Molecular mechanisms of antifibrotic effect of interferon-. g. in bleomycinmouse model of lung fibrosis: down regulation of TGF-. b. and procollagen I and III gene expression. Exp Lung Res 21:791–808
Duncan MR, Hasan A, Berman B (1995) Pentoxifylline, pentifylline, and interferons decrease type I and III procollagen mRNA levels in dermal fibroblasts: evidence for mediation by nuclear factor 1 down-regulation. J Invest Dermatol 104:282–286
Tredget ET, Wang RJ, Shen Q et al (2000) Transforming growth factor-. b. mRNA and protein in hypertrophic scar tissues and fibroblasts: antagonism by IFN-. a. and IFN-. g. in vitro and in vivo. J Interferon Cytokine Res 20:143–151
Gailit J, Clark RA (1994) Wound repair in the context of extracellular matrix. Curr Opin Cell Biol 6:717–725
McPherson JM, Piez KA (1996) Collagen in dermal wound repair. In: Clark RAF (ed) The molecular and cell biology of wound repair. Plenum, New York, pp 471–496
Rockwell WB, Cohen IK, Ehrlich HP (1989) Keloids and hypertrophic scars: a comprehensive review. Plast Reconstr Surg 84:827–837
Barbul A (1990) Immune aspects of wound repair. Clin Plast Surg 17:433–442
Barrow RE, Dasu MR (2005) Oxidative and heat stress gene changes in hypertrophic scar fibroblasts stimulated with Interleukin-1. b. J Surg Res 126:59–65
Acknowledgment
This work was supported by grants from the Hangzhou Normal University Foundation and Education Department of Zhejiang Province. We are grateful to Xielai Zhou M.D. and Professor Li Sun M.D. from Hangzhou Normal University, for advice on the manuscript and help on statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, S., Li, D., Teng, J. et al. Effects of steroids, interferon-2B, or interluekin 1B on apoptosis of fibroblasts from keloid, hypertrophic scars, and normal skin and related signal pathway. Eur J Plast Surg 30, 159–167 (2007). https://doi.org/10.1007/s00238-007-0165-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00238-007-0165-3