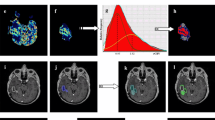
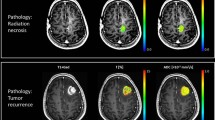
Abstract
Purpose
After standard treatment for glioblastoma, perfusion MRI remains challenging for differentiating tumor progression from post-treatment changes. Our objectives were (1) to correlate rCBV values at diagnosis and at first tumor progression and (2) to analyze the relationship of rCBV values at tumor recurrence with enhancing volume, localization of tumor progression, and time elapsed since the end of radiotherapy in tumor recurrence.
Methods
Inclusion criteria were (1) age > 18 years, (2) histologically confirmed glioblastoma treated with STUPP regimen, and (3) tumor progression according to RANO criteria > 12 weeks after radiotherapy. Co-registration of segmented enhancing tumor VOIs with dynamic susceptibility contrast perfusion MRI was performed using Olea Sphere software. For tumor recurrence, we correlated rCBV values with enhancing tumor volume, with recurrence localization, and with time elapsed from the end of radiotherapy to progression. Analyses were performed with SPSS software.
Results
Sixty-four patients with glioblastoma were included in the study. Changes in rCBV values between diagnosis and first tumor progression were significant (p < 0.001), with a mean and median decreases of 32% and 46%, respectively. Mean rCBV values were also different (p < 0.01) when tumors progressed distally (radiation field rCBV values of 1.679 versus 3.409 distally). However, changes and, therefore, low rCBV values after radiotherapy in tumor recurrence were independent of time.
Conclusion
Chemoradiation alters tumor perfusion and rCBV values may be decreased in the setting of tumor progression. Changes in rCBV values with respect to diagnosis, with low rCBV in tumor progression, are independent of time but related to the site of recurrence.


Similar content being viewed by others
Abbreviations
- DSC:
-
Dynamic susceptibility contrast
- IAUC:
-
Initial area under the curve
- IDH:
-
Isocitrate dehydrogenase
- RANO:
-
Response assessment in neuro-oncology
- S:
-
Sensitivity
- SD:
-
Standard deviation
- WHO:
-
World Health Organization
References
Shukla G, Alexander GS, Bakas S et al (2017) Advanced magnetic resonance imaging in glioblastoma: a review. Chin Clin Oncol 6(4):40. https://doi.org/10.21037/cco.2017.06.28
Abdulla S, Saada J, Johnson G et al (2015) Tumor progression or pseudoprogression? A review of post-treatment radiological appearances of glioblastoma. Clin Radiol 70(11):1299–1312. https://doi.org/10.1016/j.crad.2015.06.096
Weller M, van den Bent PM et al (2021) EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 18(3):170–186. https://doi.org/10.1038/s41571-020-00447-z
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28(11):1963–1972. https://doi.org/10.1200/JCO.2009.26.354
Zikou A, Sioka C, Alexiou G et al (2018) Radiation necrosis, pseudoprogression, pseudoresponse, and tumor recurrence: imaging challenges for the evaluation of treated gliomas. Contrast Media Mol Imaging. https://doi.org/10.1155/2018/6828396
Delgado-López PD, Riñones-Mena E, Corrales-García EM (2018) Treatment-related changes in glioblastoma: a review on the controversies in response assessment criteria and the concepts of true progression, pseudoprogression, pseudoresponse and radionecrosis. Clin Transl Oncol 20(8):939–953. https://doi.org/10.1007/s12094-017-1816-x
van Dijken BRJ, van Laar PJ, Holtman GA et al (2017) Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur Radiol 27(10):4129–4144. https://doi.org/10.1007/s00330-017-4789-9
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomized phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466. https://doi.org/10.1016/S1470-2045(09)70025-7
Boxerman JL, Schmainda KM, Weisskoff RM (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 27(4):859–867
Patel P, Baradaran H, Delgado D et al (2017) MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: a systematic review and meta-analysis. Neuro Oncol 19(1):118–127. https://doi.org/10.1093/neuonc/now148
Prager AJ, Martinez N, Beal K et al (2015) Diffusion and perfusion MRI to differentiate treatment-related changes including pseudoprogression from recurrent tumors in high-grade gliomas with histopathologic evidence. AJNR Am J Neuroradiol 36(5):877–885. https://doi.org/10.3174/ajnr.A4218
Shin KE, Ahn KJ, Choi HS et al (2014) DCE and DSC MR perfusion imaging in the differentiation of recurrent tumour from treatment-related changes in patients with glioma. Clin Radiol 69(6):e264-272. https://doi.org/10.1016/j.crad.2014.01.016
Young RJ, Gupta A, Shah AD et al (2013) MRI perfusion in determining pseudoprogression in patients with glioblastoma. Clin Imaging 37(1):41–49. https://doi.org/10.1016/j.clinimag.2012.02.016
Fatterpekar GM, Galheigo D, Narayana A et al (2012) Treatment-related change versus tumor recurrence in high-grade gliomas: a diagnostic conundrum–use of dynamic susceptibility contrast-enhanced (DSC) perfusion MRI. AJR Am J Roentgenol 198(1):19–26. https://doi.org/10.2214/AJR.11.7417
Sugahara T, Korogi Y, Tomiguchi S et al (2000) Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol 21(5):901–909
van Dijken BRJ, van Laar PJ, Smits M et al (2019) Perfusion MRI in treatment evaluation of glioblastomas: clinical relevance of current and future techniques. J Magn Reson Imaging 49(1):11–22. https://doi.org/10.1002/jmri.26306
Soni N, Ora M, Mohindra N et al (2020) Diagnostic performance of PET and perfusion-weighted imaging in differentiating tumor recurrence or progression from radiation necrosis in posttreatment gliomas: a review of literature. AJNR Am J Neuroradiol 41(9):1550–1557. https://doi.org/10.3174/ajnr.A6685
Feng A, Yuan P, Huang T et al (2022) Distinguishing tumor recurrence from radiation necrosis in treated glioblastoma using multiparametric MRI. Acad Radiol 29(9):1320–1331. https://doi.org/10.1016/j.acra.2021.11.008
Kim JY, Park JE, Jo Y et al (2019) Incorporating diffusion- and perfusion-weighted MRI into a radiomics model improves diagnostic performance for pseudoprogression in glioblastoma patients. Neuro Oncol 21(3):404–414. https://doi.org/10.1093/neuonc/noy133
Hygino da Cruz LC Jr, Rodriguez I, Domingues RC et al (2011) Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol 32(11):1978-1985. https://doi.org/10.3174/ajnr.A2397
Strauss SB, Meng A, Ebani EJ et al (2019) Imaging glioblastoma posttreatment: progression, pseudoprogression, pseudoresponse, radiation necrosis. Radiol Clin North Am 57(6):1199–1216. https://doi.org/10.1016/j.rcl.2019.07.003
Petr J, Platzek I, Seidlitz A et al (2016) Early and late effects of radiochemotherapy on cerebral blood flow in glioblastoma patients measured with non-invasive perfusion MRI. Radiother Oncol 118(1):24–28. https://doi.org/10.1016/j.radonc.2015.12.017
Seyve A, Dehais C, Chinot O et al (2023) Incidence and characteristics of pseudoprogression in IDH-mutant high-grade gliomas: a POLA network study. Neuro Oncol 25(3):495–507. https://doi.org/10.1093/neuonc/noac194
Richter V, Klose U, Bender B et al (2021) Dynamic susceptibility perfusion imaging for differentiating progressive disease from pseudoprogression in diffuse glioma molecular subtypes. J Clin Med 10(4):598. https://doi.org/10.3390/jcm10040598
Hyare H, Thust S, Rees J (2017) Advanced MRI techniques in the monitoring of treatment of gliomas. Curr Treat Options Neurol 19(3):11. https://doi.org/10.1007/s11940-017-0445-6
Funding
This work was partially supported by grants PI21/01168 and PI21/01406 from the Spanish Instituto de Salud Carlos III.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no financial or non-financial interest to disclose.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hilario, A., Salvador, E., Cardenas, A. et al. Low rCBV values in glioblastoma tumor progression under chemoradiotherapy. Neuroradiology 66, 317–323 (2024). https://doi.org/10.1007/s00234-023-03279-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-023-03279-7