Abstract
Purpose
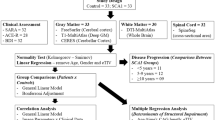
Spinocerebellar ataxia type 2 (SCA2) is a progressive neurodegenerative disorder characterized by cerebellar atrophy. However, studies to elucidate the longitudinal progression of the neuropathology are limited. We sought to identify brain macrostructural and microstructural alterations in patients with SCA2 using fixel-based analysis (FBA) to better understand its distribution patterns and progression.
Methods
We enrolled 9 patients with SCA2 and 16 age- and gender-matched controls. Longitudinal clinical and imaging data were collected at baseline, and 3.5 years later. Fiber density (FD), fiber-bundle cross-section (FC), and a combination of FD and FC (FDC) were calculated. The paired t-test was used to examine longitudinal differences. The associations between fixel-based metrics and clinical variables were explored in SCA2 patients.
Results
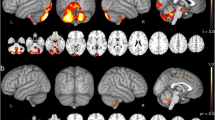
At baseline, patients with SCA2 displayed multiple white matter tracts with significantly decreased FD, FC, and FDC in the corticospinal tract, cerebellar peduncles, brainstem, corpus callosum, thalamus, striatum, and prefrontal cortex, compared to controls. Over time, many of these macrostructural and microstructural alterations progressed, manifesting lower FD, FC, and FDC in corticospinal tract, middle cerebellar peduncle, brainstem, striatum, fornix, and cingulum. No significant brain white matter alterations were found in the healthy controls over time. There was no association between the FBA-derived metrics and clinical variables in SCA2.
Conclusion
This study provides evidence of brain macrostructural and microstructural alterations and of progression over time in SCA2. The FBA-derived metrics may serve as potential biomarkers of SCA2 progression.



Similar content being viewed by others
Data availability
Data are available from a public dataset via OpenNeuro (https://openneuro.org/datasets/ds001378/versions/00003).
Code availability
None.
References
Lorenzetti D, Bohlega S, Zoghbi HY (1997) The expansion of the CAG repeat in ataxin-2 is a frequent cause of autosomal dominant spinocerebellar ataxia. Neurology 49:1009–1013
Magana JJ, Velazquez-Perez L, Cisneros B (2013) Spinocerebellar ataxia type 2: clinical presentation, molecular mechanisms, and therapeutic perspectives. Mol Neurobiol 47:90–104
Seidel K, Siswanto S, Brunt ER, den Dunnen W, Korf HW, Rub U (2012) Brain pathology of spinocerebellar ataxias. Acta Neuropathol 124:1–21
Lupo M, Olivito G, Iacobacci C, Clausi S, Romano S, Masciullo M, Molinari M, Cercignani M, Bozzali M, Leggio M (2018) The cerebellar topography of attention sub-components in spinocerebellar ataxia type 2. Cortex 108:35–49
Mascalchi M, Diciotti S, Giannelli M, Ginestroni A, Soricelli A, Nicolai E, Aiello M, Tessa C, Galli L, Dotti MT, Piacentini S, Salvatore E, Toschi N (2014) Progression of brain atrophy in spinocerebellar ataxia type 2: a longitudinal tensor-based morphometry study. PLoS One 9:e89410
Stezin A, Bhardwaj S, Khokhar S, Hegde S, Jain S, Bharath RD, Saini J, Pal PK (2021) In vivo microstructural white matter changes in early spinocerebellar ataxia 2. Acta Neurol Scand 143:326–332
Clausi S, Olivito G, Siciliano L, Lupo M, Bozzali M, Masciullo M, Molinari M, Romano S, Leggio M (2021) The neurobiological underpinning of the social cognition impairments in patients with spinocerebellar ataxia type 2. Cortex 138:101–112
Mascalchi M, Toschi N, Giannelli M, Ginestroni A, Della Nave R, Nicolai E, Bianchi A, Tessa C, Salvatore E, Aiello M, Soricelli A, Diciotti S (2015) Progression of microstructural damage in spinocerebellar ataxia type 2: a longitudinal DTI study. AJNR Am J Neuroradiol 36:1096–1101
Raffelt DA, Tournier JD, Smith RE, Vaughan DN, Jackson G, Ridgway GR, Connelly A (2017) Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage 144:58–73
Jeurissen B, Leemans A, Tournier JD, Jones DK, Sijbers J (2013) Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp 34:2747–2766
Seehaus A, Roebroeck A, Bastiani M, Fonseca L, Bratzke H, Lori N, Vilanova A, Goebel R, Galuske R (2015) Histological validation of high-resolution DTI in human post mortem tissue. Front Neuroanat 9:98
Dhollander T, Clemente A, Singh M, Boonstra F, Civier O, Duque JD, Egorova N, Enticott P, Fuelscher I, Gajamange S, Genc S, Gottlieb E, Hyde C, Imms P, Kelly C, Kirkovski M, Kolbe S, Liang X, Malhotra A et al (2021) Fixel-based analysis of diffusion MRI: methods, applications, challenges and opportunities. Neuroimage 241:118417
Adanyeguh IM, Perlbarg V, Henry PG, Rinaldi D, Petit E, Valabregue R, Brice A, Durr A, Mochel F (2018) Autosomal dominant cerebellar ataxias: imaging biomarkers with high effect sizes. Neuroimage Clin 19:858–867
Park YW, Joers JM, Guo B, Hutter D, Bushara K, Adanyeguh IM, Eberly LE, Oz G, Lenglet C (2020) Assessment of cerebral and cerebellar white matter microstructure in spinocerebellar ataxias 1, 2, 3, and 6 using diffusion MRI. Front Neurol 11:411
Filla A, DeMichele G, Caruso G, Marconi R, Campanella G (1990) Genetic data and natural history of Friedreich’s disease: a study of 80 Italian patients. J Neurol 237:345–351
Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B (1997) International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci 145:205–211
Tournier JD, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, Christiaens D, Jeurissen B, Yeh CH, Connelly A (2019) MRtrix3: a fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 202:116137
Veraart J, Fieremans E, Novikov DS (2016) Diffusion MRI noise mapping using random matrix theory. Magn Reson Med 76:1582–1593
Smith RE, Tournier JD, Calamante F, Connelly A (2013) SIFT: spherical-deconvolution informed filtering of tractograms. Neuroimage 67:298–312
Raffelt DA, Smith RE, Ridgway GR, Tournier JD, Vaughan DN, Rose S, Henderson R, Connelly A (2015) Connectivity-based fixel enhancement: whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. Neuroimage 117:40–55
Velazquez-Perez LC, Rodriguez-Labrada R, Fernandez-Ruiz J (2017) Spinocerebellar ataxia type 2: clinicogenetic aspects, mechanistic insights, and management approaches. Front Neurol 8:472
Salvatore E, Tedeschi E, Mollica C, Vicidomini C, Varrone A, Coda AR, Brunetti A, Salvatore M, De Michele G, Filla A, Pappata S (2014) Supratentorial and infratentorial damage in spinocerebellar ataxia 2: a diffusion-weighted MRI study. Mov Disord 29:780–786
Mascalchi M, Marzi C, Giannelli M, Ciulli S, Bianchi A, Ginestroni A, Tessa C, Nicolai E, Aiello M, Salvatore E, Soricelli A, Diciotti S (2018) Histogram analysis of DTI-derived indices reveals pontocerebellar degeneration and its progression in SCA2. PLoS One 13:e0200258
Moreno-Lopez Y, Olivares-Moreno R, Cordero-Erausquin M, Rojas-Piloni G (2016) Sensorimotor integration by corticospinal system. Front Neuroanat 10:24
Barthelemy D, Grey MJ, Nielsen JB, Bouyer L (2011) Involvement of the corticospinal tract in the control of human gait. Prog Brain Res 192:181–197
Ramnani N (2006) The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci 7:511–522
Welniarz Q, Dusart I, Roze E (2017) The corticospinal tract: evolution, development, and human disorders. Dev Neurobiol 77:810–829
Hernandez-Castillo CR, Vaca-Palomares I, Galvez V, Campos-Romo A, Diaz R, Fernandez-Ruiz J (2016) Cognitive deficits correlate with white matter deterioration in spinocerebellar ataxia type 2. J Int Neuropsychol Soc 22:486–491
Olivito G, Lupo M, Iacobacci C, Clausi S, Romano S, Masciullo M, Molinari M, Cercignani M, Bozzali M, Leggio M (2017) Microstructural MRI basis of the cognitive functions in patients with spinocerebellar ataxia type 2. Neuroscience 366:44–53
Kremlacek J, Valis M, Masopust J, Urban A, Zumrova A, Talab R, Kuba M, Kubova Z, Langrova J (2011) An electrophysiological study of visual processing in spinocerebellar ataxia type 2 (SCA2). Cerebellum 10:32–42
Schilling KG, Janve V, Gao Y, Stepniewska I, Landman BA, Anderson AW (2018) Histological validation of diffusion MRI fiber orientation distributions and dispersion. Neuroimage 165:200–221
Storelli L, Pagani E, Preziosa P, Filippi M, Rocca MA (2021) Measurement of white matter fiber-bundle cross-section in multiple sclerosis using diffusion-weighted imaging. Mult Scler 27:818–826
Raffelt D, Tournier JD, Rose S, Ridgway GR, Henderson R, Crozier S, Salvado O, Connelly A (2012) Apparent fibre density: a novel measure for the analysis of diffusion-weighted magnetic resonance images. Neuroimage 59:3976–3994
Mito R, Raffelt D, Dhollander T, Vaughan DN, Tournier JD, Salvado O, Brodtmann A, Rowe CC, Villemagne VL, Connelly A (2018) Fibre-specific white matter reductions in Alzheimer’s disease and mild cognitive impairment. Brain 141:888–902
Acknowledgements
Data for this study was obtained from the OpenNeuro platform (https://openneuro.org/datasets/ds001378/versions/00003). The authors thank Mascalchi M, Marzi C, Giannelli M, Ciulli S, Bianchi A, Ginestroni A, Tessa C, Nicolai E, Aiello M, Salvatore E, Soricelli A, and Diciotti S for their work on “Histogram analysis of DTI-derived indices reveals pontocerebellar degeneration and its progression in SCA2” (DOI: 10.1371/journal.pone.0200258). Their work provided valuable data and insights that significantly contributed to the findings of this study.
Funding
None.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
This research was approved by the Ethics Committee of the Careggi University Hospital of Florence, Italy.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
None of the authors of this manuscript has any potential conflict of interest related to the content of this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tu, Y., Li, Z., Xiong, F. et al. Progressive white matter degeneration in patients with spinocerebellar ataxia type 2. Neuroradiology 66, 101–108 (2024). https://doi.org/10.1007/s00234-023-03260-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-023-03260-4