Abstract
Purpose
Cerebellum modulates the amplitude of resting tremor in Parkinson’s disease (PD) via cerebello-thalamo-cortical (CTC) circuit. Tremor-related white matter alterations have been identified in PD patients by pathological studies, but in vivo evidence is limited; the influence of such cerebellar white matter alterations on tremor-related brain network, including CTC circuit, is also unclear. In this study, we investigated the cerebral and cerebellar white matter alterations in PD patients with resting tremor using diffusion tensor imaging (DTI).
Methods
In this study, 30 PD patients with resting tremor (PDWR), 26 PD patients without resting tremor (PDNR), and 30 healthy controls (HCs) from the Parkinson’s Progression Markers Initiative (PPMI) cohort were included. Tract-based spatial statistics (TBSS) and region of interest-based analyses were conducted to determine white matter difference. Correlation analysis between DTI measures and clinical characteristics was also performed.
Results
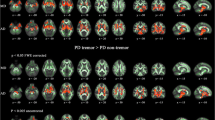
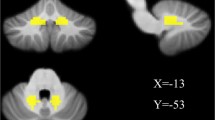
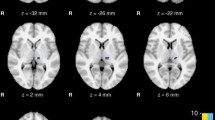
In the whole brain, TBSS and region of interest-based analyses identified higher fractional anisotropy (FA) value, lower mean diffusivity (MD) value, and lower radial diffusivity (RD) in multiple fibers. In the cerebellum, TBSS analysis revealed significantly higher FA value, decreased RD value as well as MD value in multiple cerebellar tracts including the inferior cerebellar peduncle (ICP) and middle cerebellar peduncle (MCP) when comparing the PDWR with HC, and higher FA value in the MCP when compared with PDNR.
Conclusion
We identified better white matter integrity in the cerebrum and cerebellum in PDWR indicating a potential association between the cerebral and cerebellar white matter and resting tremor in PD.




Similar content being viewed by others
Data availability
The data for the PPMI cohort is available at https://www.ppmi-info.org/access-data-specimens/download-data. All authors were approved for use of the database by PPMI study as independent researchers. All other data and codes relating to this study are available upon reasonable request to the corresponding authors.
References
Helmich RC, Hallett M, Deuschl G, Toni I, Bloem BR (2012) Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain 135(Pt 11):3206–26. https://doi.org/10.1093/brain/aws023
Zhong Y, Liu H, Liu G, Zhao L, Dai C, Liang Y et al (2022) A review on pathology, mechanism, and therapy for cerebellum and tremor in Parkinson’s disease. NPJ Parkinsons Dis 8(1):82. https://doi.org/10.1038/s41531-022-00347-2
Dirkx MF, den Ouden H, Aarts E, Timmer M, Bloem BR, Toni I et al (2016) The cerebral network of Parkinson’s tremor: an effective connectivity fMRI study. J Neurosci 36(19):5362–72. https://doi.org/10.1523/jneurosci.3634-15.2016
Luo C, Song W, Chen Q, Yang J, Gong Q, Shang HF (2017) White matter microstructure damage in tremor-dominant Parkinson’s disease patients. Neuroradiology 59(7):691–8. https://doi.org/10.1007/s00234-017-1846-7
Wen MC, Heng HSE, Lu Z, Xu Z, Chan LL, Tan EK et al (2018) Differential white matter regional alterations in motor subtypes of early drug-naive Parkinson’s disease patients. Neurorehabil Neural Repair 32(2):129–41. https://doi.org/10.1177/1545968317753075
Kuo SH, Lin CY, Wang J, Sims PA, Pan MK, Liou JY et al (2017) Climbing fiber-Purkinje cell synaptic pathology in tremor and cerebellar degenerative diseases. Acta Neuropathol 133(1):121–38. https://doi.org/10.1007/s00401-016-1626-1
Thiebaut de Schotten M, Forkel SJ (2022) The emergent properties of the connected brain. Science 378(6619):505–510. https://doi.org/10.1126/science.abq2591
Haghshomar M, Shobeiri P, Seyedi SA, Abbasi-Feijani F, Poopak A, Sotoudeh H, et al (2022). Cerebellar microstructural abnormalities in Parkinson’s disease: a systematic review of diffusion tensor imaging studies. Cerebellum. https://doi.org/10.1007/s12311-021-01355-3
Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Toni I (2011) Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol 69(2):269–81. https://doi.org/10.1002/ana.22361
Dirkx MF, Zach H, van Nuland A, Bloem BR, Toni I, Helmich RC (2019) Cerebral differences between dopamine-resistant and dopamine-responsive Parkinson’s tremor. Brain 142(10):3144–57. https://doi.org/10.1093/brain/awz261
Dirkx MF, den Ouden HE, Aarts E, Timmer MH, Bloem BR, Toni I et al (2017) Dopamine controls Parkinson’s tremor by inhibiting the cerebellar thalamus. Brain 140(3):721–34. https://doi.org/10.1093/brain/aww331
Zach H, Dirkx MF, Roth D, Pasman JW, Bloem BR, Helmich RC (2020) Dopamine-responsive and dopamine-resistant resting tremor in Parkinson disease. Neurology 95(11):e1461–e70. https://doi.org/10.1212/wnl.0000000000010316
Hall JM, Ehgoetz Martens KA, Walton CC, O’Callaghan C, Keller PE, Lewis SJ et al (2016) Diffusion alterations associated with Parkinson’s disease symptomatology: a review of the literature. Parkinsonism Relat Disord 33:12–26. https://doi.org/10.1016/j.parkreldis.2016.09.026
Pasquini J, Ceravolo R, Qamhawi Z, Lee JY, Deuschl G, Brooks DJ et al (2018) Progression of tremor in early stages of Parkinson’s disease: a clinical and neuroimaging study. Brain 141(3):811–21. https://doi.org/10.1093/brain/awx376
Buijink AWG, van Rootselaar AF, Helmich RC (2022) Connecting tremors - a circuits perspective. Curr Opin Neurol 35(4):518–24. https://doi.org/10.1097/wco.0000000000001071
van den Berg KRE, Helmich RC (2021) The role of the cerebellum in tremor - evidence from neuroimaging. Tremor Other Hyperkinet Mov (N Y) 11:49. https://doi.org/10.5334/tohm.660
Juttukonda MR, Franco G, Englot DJ, Lin YC, Petersen KJ, Trujillo P et al (2019) White matter differences between essential tremor and Parkinson disease. Neurology 92(1):e30–e9. https://doi.org/10.1212/wnl.0000000000006694
(2011) The parkinson progression marker initiative (PPMI). Prog Neurobiol 95(4):629–35. https://doi.org/10.1016/j.pneurobio.2011.09.005
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P et al (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23(15):2129–70. https://doi.org/10.1002/mds.22340
O’Gorman Tuura RL, Baumann CR, Baumann-Vogel H (2018) Beyond dopamine: GABA, glutamate, and the axial symptoms of Parkinson disease. Front Neurol 9:806. https://doi.org/10.3389/fneur.2018.00806
Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC (2013) How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov Disord 28(5):668–70. https://doi.org/10.1002/mds.25383
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW (2012) Smith SM (2011). FSL Neuroimage 62(2):782–790. https://doi.org/10.1016/j.neuroimage.2011.09.015
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1):S208-19. https://doi.org/10.1016/j.neuroimage.2004.07.051
Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS et al (2008) Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 39(1):336–47. https://doi.org/10.1016/j.neuroimage.2007.07.053
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31(4):1487–505. https://doi.org/10.1016/j.neuroimage.2006.02.024
Nicoletti G, Fera F, Condino F, Auteri W, Gallo O, Pugliese P et al (2006) MR imaging of middle cerebellar peduncle width: differentiation of multiple system atrophy from Parkinson disease. Radiology 239(3):825–30. https://doi.org/10.1148/radiol.2393050459
van Baarsen KM, Kleinnijenhuis M, Jbabdi S, Sotiropoulos SN, Grotenhuis JA, van Cappellen van Walsum AM (2015) A probabilistic atlas of the cerebellar white matter. NeuroImage 124:724–32. https://doi.org/10.1016/j.neuroimage.2015.09.014
Herweh C, Akbar M, Wengenroth M, Heiland S, Bendszus M, Stippich C (2010) Reduced anisotropy in the middle cerebellar peduncle in Chiari-II malformation. Cerebellum 9(3):303–9. https://doi.org/10.1007/s12311-010-0162-0
Lenka A, Ingalhalikar M, Shah A, Saini J, Arumugham SS, Hegde S et al (2020) Abnormalities in the white matter tracts in patients with Parkinson disease and psychosis. Neurology 94(18):e1876–e84. https://doi.org/10.1212/wnl.0000000000009363
Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL et al (2007) Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36(3):630–44. https://doi.org/10.1016/j.neuroimage.2007.02.049
Jia X-Z, Zhao N, Dong H-M, Sun J-W, Barton M, Burciu R et al (2021) Small P values may not yield robust findings: an example using REST-meta-PD. Sci Bull 66(21):2148–52. https://doi.org/10.1016/j.scib.2021.06.007
Abdelgabar AR, Suttrup J, Broersen R, Bhandari R, Picard S, Keysers C et al (2019) Action perception recruits the cerebellum and is impaired in patients with spinocerebellar ataxia. Brain 142(12):3791–805. https://doi.org/10.1093/brain/awz337
Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N (2009) A probabilistic MR atlas of the human cerebellum. Neuroimage 46(1):39–46. https://doi.org/10.1016/j.neuroimage.2009.01.045
Novellino F, Nicoletti G, Cherubini A, Caligiuri ME, Nisticò R, Salsone M et al (2016) Cerebellar involvement in essential tremor with and without resting tremor: a diffusion tensor imaging study. Parkinsonism Relat Disord 27:61–6. https://doi.org/10.1016/j.parkreldis.2016.03.022
Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed 15(7–8):435–55. https://doi.org/10.1002/nbm.782
D’Angelo E (2018) Physiology of the cerebellum. Handb Clin Neurol 154:85–108. https://doi.org/10.1016/b978-0-444-63956-1.00006-0
Milosevic L, Kalia SK, Hodaie M, Lozano AM, Popovic MR, Hutchison WD (2018) Physiological mechanisms of thalamic ventral intermediate nucleus stimulation for tremor suppression. Brain 141(7):2142–55. https://doi.org/10.1093/brain/awy139
D’Angelo E, Casali S (2012) Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front Neural Circuits 6:116. https://doi.org/10.3389/fncir.2012.00116
Bostan AC, Strick PL (2018) The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci 19(6):338–50. https://doi.org/10.1038/s41583-018-0002-7
Sweet JA, Walter BL, Gunalan K, Chaturvedi A, McIntyre CC, Miller JP (2014) Fiber tractography of the axonal pathways linking the basal ganglia and cerebellum in Parkinson disease: implications for targeting in deep brain stimulation. J Neurosurg 120(4):988–96. https://doi.org/10.3171/2013.12.Jns131537
Meng F, Hu W, Wang S, Tam J, Gao Y, Zhu XL, et al (2023). Utilization, surgical populations, centers, coverages, regional balance, and their influential factors of deep brain stimulation for Parkinson’s disease: a large-scale multicenter cross-sectional study from 1997–2021. Int J Surg. https://doi.org/10.1097/js9.0000000000000603
Mure H, Hirano S, Tang CC, Isaias IU, Antonini A, Ma Y et al (2011) Parkinson’s disease tremor-related metabolic network: characterization, progression, and treatment effects. Neuroimage 54(2):1244–53. https://doi.org/10.1016/j.neuroimage.2010.09.028
Gonzalez-Escamilla G, Muthuraman M, Ciolac D, Coenen VA, Schnitzler A, Groppa S (2020) Neuroimaging and electrophysiology meet invasive neurostimulation for causal interrogations and modulations of brain states. Neuroimage 220:117144. https://doi.org/10.1016/j.neuroimage.2020.117144
Wang S, Zhu G, Shi L, Zhang C, Wu B, Yang A et al (2023) Closed-loop adaptive deep brain stimulation in Parkinson’s disease: procedures to achieve it and future perspectives. J Parkinsons Dis 13(4):453–71. https://doi.org/10.3233/jpd-225053
Acknowledgements
This study is supported by National Natural Science Foundation of China (grant number: 82001367, receiver: Xi Liu), Natural Science Foundation of Chongqing (grant number: cstc2021jcyj-msxmX0180, receiver: Xi Liu), Kuanren Talent Program of The Second Affiliated Hospital of Chongqing Medical University (receiver: Xi Liu), and Graduate Students Science Innovation Project of Chongqing (grant number: CYS22349, receiver: Yuke Zhong).
Funding
This study is supported by National Natural Science Foundation of China (grant number: 82001367, receiver: Xi Liu), Natural Science Foundation of Chongqing (grant number: cstc2021jcyj-msxmX0180, receiver: Xi Liu), Kuanren Talent Program of The Second Affiliated Hospital of Chongqing Medical University (receiver: Xi Liu), and Graduate Students Science Innovation Project of Chongqing (grant number: CYS22349, receiver: Yuke Zhong).
Author information
Authors and Affiliations
Contributions
L.C., F.D., and X.L.: conception and design; Y.Z., H.L., H.L., and G.L.: data screening; Y.Z., L.Z., C.D., and Y.L.: image processing and statistical analysis; Y.Z.: first draft; Y.Z., L.M., C.T., and X.L.: review and editing. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no financial or non-financial interest.
Ethics approval and informed consent
The PPMI study has been approved by the institutional review boards, and written informed consent was provided by all subjects. Details are available at https://www.ppmi-info.org/access-data-specimens/download-data.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhong, Y., Liu, H., Liu, G. et al. Cerebellar and cerebral white matter changes in Parkinson’s disease with resting tremor. Neuroradiology 65, 1497–1506 (2023). https://doi.org/10.1007/s00234-023-03206-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-023-03206-w