Abstract
Purpose
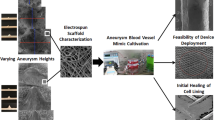
Neurothrombectomy catheters can disrupt or injure the vessel wall. This potential injury is often studied in animal or cadaver models, but prior work suggests that endothelialized silicone models may be an option for early in vitro assessment. The purpose of this work was to create a complex, clinically-relevant endothelialized neurovascular silicone model, and to determine the utility of the model for evaluating vessel injury due to catheter simulated use.
Methods
Models of the ICA and MCA were fabricated out of silicone, sterilized, coated with fibronectin, placed in bioreactors, and endothelialized with HUVECs. These silicone vessels were maintained under flow for 3 and 7 days, and cellular linings were assessed. Subsequently, 24 silicone vessels were created and treated with neurovascular catheters. Vessels were accessed with a guidewire, microcatheter, and/or aspiration catheter, either once (1-pass) or three times (3-pass). Vessels were then fixed, and injury was evaluated through quantitative image analysis and a visual scoring system.
Results
Complex silicone models were successfully endothelialized and maintained with consistent cell linings. The transparent silicone permitted catheter simulated use without fluoroscopy, and injury to the vessel wall was observed and successfully imaged and characterized. Vessels subjected to 3-passes exhibited more injury than 1-pass, and injury increased with the number and size of devices. These results illustrated expected trends and support use of these models for early assessment of vessel injury.
Conclusion
Complex silicone neurovascular models can be endothelialized and used in vitro to assess and compare injury due to the use of neurovascular catheters.




Similar content being viewed by others
References
Lv X (2021) The history and development of endovascular neurosurgery. In: Frontiers in Clinical Neurosurgery. IntechOpen
Prabhakaran S, Ruff I, Bernstein RA (2015) Acute stroke intervention. JAMA 313:1451
Nogueira RG, Lutsep HL, Gupta R et al (2012) Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet 380:1231–1240
Guglielmi G (2007) History of endovascular endosaccular occlusion of brain aneurysms: 1965-1990. Interv Neuroradiol 13:217–224
Hidaka M, Yamaguchi S, Koyanagi Y, Arakawa S (2019) Reocclusion of the treated vessel due to endothelial injury after mechanical thrombectomy in a patient with acute ischaemic stroke. BMJ Case Rep 12:e228937
Liu Y, Abbasi M, Arturo Larco JL et al (2021) Preclinical testing platforms for mechanical thrombectomy in stroke: a review on phantoms, in-vivo animal, and cadaveric models. J Neurointerv Surg 13:816–822
Chueh JY, Wakhloo AK, Gounis MJ (2009) Neurovascular Modeling: Small-Batch Manufacturing of Silicone Vascular Replicas. Am J Neuroradiol 30:1159–1164
Sommer KN, Bhurwani MMS, Tutino V et al (2021) Use of patient specific 3D printed neurovascular phantoms to simulate mechanical thrombectomy. 3D Print Med 7:32
Reddy AS, Liu Y, Cockrum J et al (2021) Construction of a comprehensive endovascular test bed for research and device development in mechanical thrombectomy in stroke. J Neurosurg 134:1190–1197
Teng D, Pannell JS, Rennert RC et al (2015) Endothelial Trauma From Mechanical Thrombectomy in Acute Stroke. Stroke 46:1099–1106
Carniato S, Mehra M, King RM et al (2013) Porcine Brachial Artery Tortuosity for In Vivo Evaluation of Neuroendovascular Devices. Am J Neuroradiol 34:E36–E38
Georganos SA, Guilbert F, Salazkin I et al (2004) Surgical construction of an in vivo carotid siphon model to test neurovascular devices. Neurosurgery 54:1239–1243
Gao B-L, Wang Y-L, Zhang X-J et al (2017) Construction of an in vivo carotid siphon model for testing endovascular devices for neuro-interventions. Interv Neuroradiol 23:325–329
Gory B, Bresson D, Rouchaud A et al (2013) A novel swine model to evaluate arterial vessel injury after mechanical endovascular thrombectomy. Interv Neuroradiol 19:147–152
Peschillo S, Tomasello A, Diana F et al (2018) Comparison of subacute vascular damage caused by adapt versus stent retriever devices after thrombectomy in acute ischemic stroke: histological and ultrastructural study in an animal model. Interv Neurol 7:501–512
Li C-H, Gao B-L, Wang J-W et al (2019) Construction of aneurysmal models on a curved vascular segment of a carotid siphon model for testing endovascular devices. World Neurosurg 123:e581–e587
Arai D, Ishii A, Chihara H et al (2016) Histological examination of vascular damage caused by stent retriever thrombectomy devices. J Neurointerv Surg 8:992–995
Arturo Larco JL, Madhani SI, Liu Y et al (2023) Human “live cadaver” neurovascular model for proximal and distal mechanical thrombectomy in stroke. J Neurointerv Surg 15:465–472
Villadolid C, Puccini B, Dennis B et al (2020) Custom tissue engineered aneurysm models with varying neck size and height for early stage in vitro testing of flow diverters. J Mater Sci Mater Med 31:34
McCulloch A, Turcott A, Graham G et al (2021) Endothelialized silicone aneurysm models for in vitro evaluation of flow diverters. J Neurointerv Surg 13:727–731
Sugiu K, Martin J-B, Jean B et al (2003) Artificial cerebral aneurysm model for medical testing, training, and research. Neurol Med Chir 43:69–72 discussion 73
Thompson JW, Elwardany O, McCarthy DJ et al (2019) In vivo cerebral aneurysm models. Neurosurg Focus 47:E20
Kent KC, Shindo S, Ikemoto T, Whittemore AD (1989) Species variation and the success of endothelial cell seeding. J Vasc Surg 9:271–276
Sharefkin JB, van Wart HE, Cruess DF et al (1986) Adult human endothelial cell enzymatic harvesting. Estimates of efficiency and comparison of crude and partially purified bacterial collagenase preparations by replicate microwell culture and fibronectin degradation measured by enzyme-linked immunosorbent assay. J Vasc Surg 4:567–577
Fitzgerald S, Ryan D, Thornton J, Nogueira RG (2021) Preclinical evaluation of Millipede 088 intracranial aspiration catheter in cadaver and in vitro thrombectomy models. J Neurointerv Surg 13:447–452
Mejia J, Gutierrez J, Patino Hoyos M et al (2020) E-130 Step forward: early experience using OCT technology in neurovascular field. In: Electronic Poster Abstracts. BMJ Publishing Group Ltd., BMA House, Tavistock Square, London, WC1H 9JR, p A99.1-A99
Acknowledgements
We would like to acknowledge Harrison Oen and Jack Dooley for their assistance with the extensive endothelial cell culture in these studies, and also Jonah Adams and Jake Perisho for their assistance with the confirmatory PECAM-1 staining in the catheter simulated use vessels. We would like to acknowledge Sarena Carniato for her thoughtful review and feedback on the written manuscript.
Funding
This work was funded by Stryker Neurovascular.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares a conflict of interest (see stated author affiliations and funding).
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
N/A
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McCulloch, A., Yang, B., Frenklakh, S. et al. Evaluation of vessel injury after simulated catheter use in an endothelialized silicone model of the intracranial arteries. Neuroradiology 65, 1507–1515 (2023). https://doi.org/10.1007/s00234-023-03197-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-023-03197-8