Abstract
Purpose
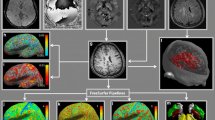
Studies on hypothalamic changes in patients with relapsing remitting multiple sclerosis (RRMS) are very scarce, despite the fact that the relationship with the hypothalamus is frequently reported. The aim of the study was to determine the volume of the hypothalamic subunits and the total hypothalamus and its relationship with the total demyelinating lesion volume (TLV) and expanded disability status scale (EDSS) in RRMS patients.
Methods
In this cross-sectional study, anterior–superior, superior tubular, posterior hypothalamus, anterior-inferior, inferior tubular subunits of hypothalamus, and total hypothalamus volume were calculated, with fully automatic analysis methods using volumetric T1 images of 65 relapsed RRMS patients and 68 healthy controls (HC). Volume changes in the hypothalamus and its subunits in RRMS patients were examined using multivariate analysis of covariance (MANCOVA). The relationship of these volumes with EDSS and TLV was investigated by partial correlation analysis.
Results
There is volume reduction in total hypothalamus (F = 13.87, p < 0.001), anterior–superior (F = 19.2, p < 0.001), superior tubular (F = 10.1, p = 0.002) subunits, and posterior hypothalamus (F = 19.2, p < 0.001) volume in RRMS patients. EDSS correlates negatively with anterior–superior (p = 0.017, r = − 0.333), superior tubular subunits (p = 0.023, r = − 0.439), posterior hypothalamus (p < 0.001, r = − 0.511), and whole hypothalamus volume (p = 0.001, r = − 0.439). TLV correlates negatively with anterior superior (p < 0.001, r = − 0.565), anterior inferior (p = 0.002, r = − 0.431), superior tubular subunits (p = 0.002, r = − 0.432), posterior hypothalamus (p < 0.001, r = − 0.703), and whole hypothalamus (p < 0.001, r = − 0.627) volumes.
Conclusion
This study demonstrates a reduction in total hypothalamus volume, anterior–superior, superior tubular, and posterior hypothalamus in patients with RRMS. Anterior–superior and superior tubular subunit, posterior hypothalamus, and total hypothalamus volume were negatively correlated with TLV and EDSS scores.



Similar content being viewed by others
References
Najafi MR, Toghianifar N, Etemadifar M, Haghighi S, Maghzi AH, Akbari M (2013) Circadian rhythm sleep disorders in patients with multiple sclerosis and its association with fatigue: A case-control study. J Res Med Sci 18(Suppl 1):S71–S73
Davis S, Wilson T, White A, Frohman E (2010) Thermoregulation in multiple sclerosis. J Appl Physiol 109:1531–1537. https://doi.org/10.1152/japplphysiol.00460.2010.-Multiple
Haensch C, Jörg J (2006) Autonomic dysfunction in multiple sclerosis. J Neurol 253(1 SUPPL.). https://doi.org/10.1007/s00415-006-1102-2
Huitinga I, Erkut ZA, van Beurden D, Swaab DF (2004) Impaired hypothalamus-pituitary-adrenal axis activity and more severe multiple sclerosis with hypothalamic lesions. Ann Neurol 55(1):37–45. https://doi.org/10.1002/ana.10766
Ysrraelit MC, Gaitán MI, Lopez AS, Correale J (2008) Impaired hypothalamic-pituitary-adrenal axis activity in patients with multiple sclerosis. Neurology 71(24):1948–54. https://doi.org/10.1212/01.wnl.0000336918.32695.6b
Burfeind K, Yadav V, Marks D (2016) Hypothalamic dysfunction and multiple sclerosis: implications for fatigue and weight dysregulation. Curr Neurol Neurosci Rep 16(11). https://doi.org/10.1007/s11910-016-0700-3
Qiu W, Raven S, Wu J et al (2011) Hypothalamic lesions in multiple sclerosis. J Neurol Neurosurg Psychiatry 82(7):819–822. https://doi.org/10.1136/jnnp.2009.198192
Huitinga I, Erkut ZA, van Beurden D, Swaab DF (2004) Impaired hypothalamus-pituitary-adrenal axis activity and more severe multiple sclerosis with hypothalamic lesions. Ann Neurol 55(1):37–45. https://doi.org/10.1002/ana.1076
Nakashima I, Fujihara K, Miyazawa I et al (2006) Clinical and MRI features of Japanese patients with multiple sclerosis positive for NMO-IgG. J Neurol Neurosurg Psychiatry 77(9):1073–1075. https://doi.org/10.1136/jnnp.2005.080390
Polacek H, Kantorova E, Hnilicova P, Grendar M, Zelenak K, Kurca E (2019) Increased glutamate and deep brain atrophy can predict the severity of multiple sclerosis. Biomedical Papers 163(1):45–53. https://doi.org/10.5507/bp.2018.036
Kantorová E, Poláček H, Bittšanský M et al (2017) Hypothalamic damage in multiple sclerosis correlates with disease activity, disability, depression, and fatigue. Neurol Res 39(4):323–330. https://doi.org/10.1080/01616412.2016.1275460
Hnilicová P, Kantorová E, Poláček H et al (2019) Altered hypothalamic metabolism in early multiple sclerosis – MR spectroscopy study. J Neurol Sci 407. https://doi.org/10.1016/j.jns.2019.116458
Zellini F, Niepel G, Tench C, Constantinescu CS (2009) Hypothalamic involvement assessed by T1 relaxation time in patients with relapsing - remitting multiple sclerosis. Mult Scler 15(12):1442–1449. https://doi.org/10.1177/1352458509350306
Hanken K, Eling P, Kastrup A, Klein J, Hildebrandt H (2015) Integrity of hypothalamic fibers and cognitive fatigue in multiple sclerosis. Mult Scler Relat Disord 4(1):39–46. https://doi.org/10.1016/j.msard.2014.11.006
Billot B, Bocchetta M, Todd E, Dalca Av, Rohrer J, Iglesias JE (2020) Automated segmentation of the hypothalamus and associated subunits in brain MRI. Neuroimage 223. https://doi.org/10.1016/j.neuroimage.2020.117287
Cuschieri S (2019) The STROBE guidelines. Saudi J Anaesth 13(5):S31–S34. https://doi.org/10.4103/sja.SJA_543_18
Thompson A, Banwell B, Barkhof F et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17(2):162–173. https://doi.org/10.1016/S1474-4422(17)30470-2
Gaser C, Dahnke R, Thompson P, Kurth F, Luders E, Alzheimer’s Disease Neuroimaging Initiative (2022) Title of the paper: CAT-A computational anatomy toolbox for the analysis of structural MRI data. bioRxiv. https://doi.org/10.1101/2022.06.11.495736
Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26(3):839–851. https://doi.org/10.1016/j.neuroimage.2005.02.018
Schmidt P, Gaser C, Arsic M et al (2012) An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage 59(4):3774–3783. https://doi.org/10.1016/j.neuroimage.2011.11.032
Luo W, Fang L, Wang Z et al (2021) Hypothalamic lesions in neuromyelitis optica spectrum disorders: exploring a scoring system based on magnetic resonance imaging. Jpn J Radiol 39(7):659–668. https://doi.org/10.1007/s11604-021-01104-w
Rothhaas R, Chung S (2021) Role of the preoptic area in sleep and thermoregulation. Front Neurosci 15. https://doi.org/10.3389/fnins.2021.664781
Sumowski J, Leavitt V (2014) Body temperature is elevated and linked to fatigue in relapsing-remitting multiple sclerosis, even without heat exposure. Arch Phys Med Rehabil 95(7):1298–1302. https://doi.org/10.1016/j.apmr.2014.02.004
Berti F, Arif Z, Constantinescu C, Gran B (2018) Hypothermia in multiple sclerosis: beyond the hypothalamus? A case report and review of the literature. Case Rep Neurol Med 2018:1–16. https://doi.org/10.1155/2018/2768493
Zhang Y, Dong R, Fan H, Li S, Geng D (2011) Hypothalamus syndrome in opticospinal multiple sclerosis. Am J Neuroradiol 32(8). https://doi.org/10.3174/ajnr.A2278
Bamer AM, Johnson KL, Amtmann D, Kraft G (2008) Prevalence of sleep problems in individuals with multiple sclerosis. Mult Scler 14(8):1127–1130. https://doi.org/10.1177/1352458508092807
Carnická Z, Kollár B, Šiarnik P, Krížová L, Klobucníková K, Turcáni P (2015) Sleep disorders in patients with multiple sclerosis. J Clin Sleep Med 11(5):553–557. https://doi.org/10.5664/jcsm.4702
Nitz D, Siegel J (1996) GABA release in posterior hypothalamus across sleep-wake cycle. Am J Physiol Regul Integr Comp Physiol 271(6):40–6. https://doi.org/10.1152/ajpregu.1996.271.6.r1707
Foschi M, Rizzo G, Liguori R et al (2019) Sleep-related disorders and their relationship with MRI findings in multiple sclerosis. Sleep Med 56:90–97. https://doi.org/10.1016/j.sleep.2019.01.010
Daviu N, Füzesi T, Rosenegger D et al (2020) Paraventricular nucleus CRH neurons encode stress controllability and regulate defensive behavior selection. Nat Neurosci 23(3):398–410. https://doi.org/10.1038/s41593-020-0591-0
Kern S, Krause I, Horntrich A, Thomas K, Aderhold J, Ziemssen T (2013) Cortisol awakening response is linked to disease course and progression in multiple sclerosis. PLoS One 8(4). https://doi.org/10.1371/journal.pone.0060647
Hanken K, Eling P, Hildebrandt H (2014) The representation of inflammatory signals in the brain - a model for subjective fatigue in multiple sclerosis. Front Neurol 5(DEC). https://doi.org/10.3389/fneur.2014.00264
Hanken K, Manousi A, Klein J, Kastrup A, Eling P, Hildebrandt H (2016) On the relation between self-reported cognitive fatigue and the posterior hypothalamic-brainstem network. Eur J Neurol 23(1):101–109. https://doi.org/10.1111/ene.12815
Dineen R, Bradshaw C, Constantinescu C, Auer D (2012) Extra-hippocampal subcortical limbic involvement predicts episodic recall performance in multiple sclerosis. PLoS One 7(10). https://doi.org/10.1371/journal.pone.0044942
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Contributions
BG: project development, data collection, data analysis, and manuscript writing/editing. SŞ: project development, data collection, and manuscript writing/editing. KA: data collection, data analysis, and manuscript editing. Lİ: project development, data collection, and manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Genç, B., Şen, S., Aslan, K. et al. Volumetric changes in hypothalamic subunits in patients with relapsing remitting multiple sclerosis. Neuroradiology 65, 899–905 (2023). https://doi.org/10.1007/s00234-023-03122-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-023-03122-z