Abstract
Purpose
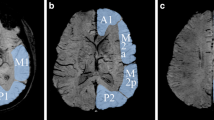
This study aimed to observe the changes of venous continuity using the susceptibility weighted imaging-minimum intensity projection (SWI-MinIP) images in children with primary headache.
Methods
The headache types were classified following the International Headache Society’s diagnostic criteria. Patients with secondary headaches were excluded. The presence of asymmetric vasculature in SWI-MinIP images was visually assessed. Moreover, the relationship between headache patterns and asymmetric hypointense signals was analyzed.
Results
In this single-center, retrospective study from 2016 to 2020, among 251 cases of primary headache (male/female, 108/143; mean age, 11.4 ± 4.0 years), 137 (54.6%), 75 (29.9%), and 39 (15.5%) patients had migraine, tension-type headache, and other primary headaches, respectively. On SWI-MinIP images, 14 (5.6%) patients showed an asymmetric venous pattern. All patients with SWI-MinIP asymmetry were included in the migraine group, accounting for 10.2% of patients with migraine. Five (35.7%) and nine (64.3%) patients were included in the aura and non-aura groups, respectively, without a significant difference in the frequency of asymmetric hypointense signals between the two groups (p = 0.325). All 14 patients with asymmetric hypervascularity had brain MRI within 12 h of headache onset. Ten (71.4%) of the 14 patients showed consistency between the laterality of headache and the hemisphere of predominant vascularity in SWI-MinIP.
Conclusion
Patients with migraine had increased cerebral venous perfusion in the most involved region of the headache on the SWI-MinIP view on a 3.0 T scanner, which can be used as a qualitative indicator with low sensitivity and high specificity for the diagnosis of primary headache in the acute phase (< 12 h).

Similar content being viewed by others
References
Watts ME, Pocock R, Claudianos C (2018) Brain energy and oxygen metabolism: emerging role in normal function and disease. Front Mol Neurosci 11:216. https://doi.org/10.3389/fnmol.2018.00216
Leonhardt G, De Greiff A, Weber J, Ludwig T, Wiedemayer H, Forsting M, Hufnagel A (2005) Brain perfusion following single seizures. Epilepsia 46:1943–1949. https://doi.org/10.1111/j.1528-1167.2005.00336.x
Cobb-Pitstick KM, Munjal N, Safier R, Cummings DD, Zuccoli G (2018) Time course of cerebral perfusion changes in children with migraine with aura mimicking stroke. AJNR Am J Neuroradiol 39:1751–1755. https://doi.org/10.3174/ajnr.A5693
Soriani S, Feggi L, Battistella PA, Arnaldi C, De Carlo L, Stipa S (1997) Interictal and ictal phase study with Tc 99 m HMPAO brain SPECT in juvenile migraine with aura. Headache 37:31–36. https://doi.org/10.1046/j.1526-4610.1997.3701031.x
Hansen JM, Schankin CJ (2019) Cerebral hemodynamics in the different phases of migraine and cluster headache. J Cereb Blood Flow Metab 39:595–609. https://doi.org/10.1177/0271678X17729783
Asghar MS, Hansen AE, Kapijimpanga T, van der Geest RJ, van der Koning P, Larsson HB, Olesen J, Ashina M (2010) Dilation by CGRP of middle meningeal artery and reversal by sumatriptan in normal volunteers. Neurology 75:1520–1526. https://doi.org/10.1212/WNL.0b013e3181f9626a
Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H (2009) Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61:283–357. https://doi.org/10.1124/pr.109.001370
Charles A (2012) Migraine is not primarily a vascular disorder. Cephalalgia 32:431–432. https://doi.org/10.1177/0333102412441717
Dooley J (2009) The evaluation and management of paediatric headaches. Paediatr Child Health 14:24–30. https://doi.org/10.1093/pch/14.1.24
Lewis DW (2002) Headaches in children and adolescents. Am Fam Physician 65:625. https://doi.org/10.1016/j.cppeds.2007.03.003
Schwedt TJ, Chiang CC, Chong CD, Dodick DW (2015) Functional MRI of migraine. Lancet Neurol 14:81–91. https://doi.org/10.1016/S1474-4422(14)70193-0
Kim H, Lee S, Lee A (2019) Examining the difference between migraine and intracranial arterial stenosis based on the transcranial doppler findings. J Neurosonol Neuroimag 11:132–138. https://doi.org/10.31728/jnn.2019.00061
Oberndorfer S, Wöber C, Nasel C, Asenbaum S, Lahrmann H, Fueger B, Grisold W (2004) Familial hemiplegic migraine: follow-up findings of diffusion-weighted magnetic resonance imaging (MRI), perfusion-MRI and [99mTc] HMPAO-SPECT in a patient with prolonged hemiplegic aura. Cephalalgia 24:533–539. https://doi.org/10.1111/j.1468-2982.2003.00706.x
Schweser F, Deistung A, Lehr BW, Reichenbach JR (2010) Differentiation between diamagnetic and paramagnetic cerebral lesions based on magnetic susceptibility mapping. Med Phys 37:5165–5178. https://doi.org/10.1118/1.3481505
Tong KA, Ashwal S, Obenaus A, Nickerson JP, Kido D, Haacke EM (2008) Susceptibility-weighted MR imaging: a review of clinical applications in children. AJNR Am J Neuroradiol 29:9–17. https://doi.org/10.3174/ajnr.A0786
Haller S, Haacke EM, Thurnher MM, Barkhof F (2021) Susceptibility-weighted imaging: technical essentials and clinical neurologic applications. Radiology 299:3–26. https://doi.org/10.1148/radiol.2021203071
Elnekeidy AE, Yehia A, Elfatatry A (2014) Importance of susceptibility weighted imaging (SWI) in management of cerebro-vascular strokes (CVS). Alex J Med 50:83–91. https://doi.org/10.1016/j.ajme.2013.05.006
Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng Y-CN (2009) Susceptibility-weighted imaging: technical aspects and clinical applications, Part 1. Am J Neuroradiol 30:19–30. https://doi.org/10.3174/ajnr.A1400
Hingwala D, Kesavadas C, Thomas B, Kapilamoorthy TR (2010) Clinical utility of susceptibility-weighted imaging in vascular diseases of the brain. Neurol India 58:602–607. https://doi.org/10.4103/0028-3886.68667
Tong KA, Ashwal S, Obenaus A, Nickerson JP, Kido D, Haacke EM (2008) Susceptibility-weighted mr imaging: a review of clinical applications in children. Am J Neuroradiol 29:9–17. https://doi.org/10.3174/ajnr.A0786
Reichenbach JR, Venkatesan R, Yablonsky DA, Thompson MR, Lai S, Haacke EM (1997) Theory and application of static field inhomogenity effects inn gradient echo imaging. J Magn Reson Imaging 7:266–279. https://doi.org/10.1002/jmri.1880070203
Liu C, Li W, Tong KA, Yeom KW, Kuzminski S (2015) Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J Magn Reson Imaging 42:23–41. https://doi.org/10.1002/jmri.24768
Kim HS, Kim SJ, Kong YH (2019) Clinical significance of asymmetric minimum intensity projection images of brain magnetic resonance imaging in children. J Clin Neurol 15:347–352. https://doi.org/10.3988/jcn.2019.15.3.347
Gocmen R, Gunbey C, Arsava EM, Oguz KK, Haliloglu G (2016) Susceptibility-weighted magnetic resonance imaging findings of two pediatric migraine patients with aura. Neuropediatrics 47:46–50. https://doi.org/10.1055/s-0035-1570322
Fedak EM, Zumberge NA, Heyer GL (2013) The diagnostic role for susceptibility-weighted MRI during sporadic hemiplegic migraine. Cephalalgia 33:1258–1263. https://doi.org/10.1177/0333102413491027
Breiding PS, Kellner-Weldon F, Grunder L, Scutelnic A, Fischer U, Meinel TR, Slavova N, Gralla J, El-Koussy M, Denier N (2020) Quantification of cerebral veins in patients with acute migraine with aura: a fully automated quantification algorithm using susceptibility-weighted imaging. PLoS One 15:e0233992. https://doi.org/10.1371/journal.pone.0233992
Kellner-Weldon F, Jossen M, Breiding PS, Grunder L, Schankin C, Scutelnic A, Fischer U, Muri R, Pastore-Wapp M, Wiest R, El-Koussy M (2021) Imaging neurovascular uncoupling in acute migraine with aura with susceptibility weighted imaging. Clin Neuroradiol 31:581–588. https://doi.org/10.1007/s00062-020-00962-7
Karaarslan E, Ulus S, Kürtüncü M (2011) Susceptibility-weighted imaging in migraine with aura. Am J Neuroradiol 32:E5–E7. https://doi.org/10.3174/ajnr.A1973
Miller C, Goldberg MF (2012) Susceptibility-weighted imaging and computed tomography perfusion abnormalities in diagnosis of classic migraine. Emerg Radiol 19:565–569. https://doi.org/10.1007/s10140-012-1051-2
Mourand I, Menjot de Champfleur N, Carra-Dallière C, Le Bars E, Roubertie A, Bonafé A et al (2012) Perfusion-weighted MR imaging in persistent hemiplegic migraine. Neuroradiology 54:255–260. https://doi.org/10.1007/s00234-011-0946-z
Schwedt TJ, Dodick DW (2009) Advanced neuroimaging of migraine. Lancet Neurol 8:560–568. https://doi.org/10.1016/S1474-4422(09)70107-3
Welch KM, Nagesh V, Aurora SK, Gelman N (2001) Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache 41:629–637. https://doi.org/10.1046/j.1526-4610.2001.041007629.x
Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, Becerra L, Borsook D (2010) Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol 68:81–91. https://doi.org/10.1002/ana.21994
Sprenger T, Borsook D (2012) Migraine changes the brain–neuroimaging imaging makes its mark. Curr Opin Neurol 25:252–262. https://doi.org/10.1097/WCO.0b013e3283532ca3
Floery D, Vosko MR, Fellner FA, Fellner C, Ginthoer C, Gruber F, Ransmayr G, Doerfler A, Uder M, Bradley WG (2012) Acute-onset migrainous aura mimicking acute stroke: MR perfusion imaging features. Am J Neuroradiol 33:1546–1552. https://doi.org/10.3174/ajnr.A3020
Rotstein DL, Aviv RI, Murray BJ (2012) Migraine with aura associated with unilateral cortical increase in vascular permeability. Cephalalgia Int J Headache 32:1216–1219. https://doi.org/10.1177/0333102412462286
Olesen J, Friberg L, Olsen TS, Iversen HK, Lassen NA, Andersen AR, Karle A (1990) Timing and topography of cerebral blood flow, aura, and headache during migraine attacks. Ann Neurol 28:791–798. https://doi.org/10.1002/ana.410280610
Andersson JL, Muhr C, Lilja A, Valind S, Lundberg PO, Långström B (1997) Regional cerebral blood flow and oxygen metabolism during migraine with and without aura. Cephalalgia Int J Headache 17:570–579. https://doi.org/10.1046/j.1468-2982.1997.1705570.x
Acknowledgements
None.
Funding
The authors (Han MJ, Park SY, Hwang SB, and Kim SJ) declare that no party has direct interests in the results of this study and that no organization with which the authors are associated has offered or will confer a benefit to them concerning the results of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the Institutional Review Board of Jeonbuk National University Hospital (IRB no. 2020–03-032).
Informed consent
The need to obtain informed consent was waived.
Conflict of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, M.J., Park, S.Y., Hwang, S. et al. Clinical significance of asymmetric hypointense signals in minimum intensity projections of brain magnetic resonance imaging in children with primary headache. Neuroradiology 65, 415–422 (2023). https://doi.org/10.1007/s00234-022-03076-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-03076-8