Abstract
Purpose
To investigate whether texture features from tumor and peritumoral areas based on sequence combinations can differentiate between low- and non-low-grade meningiomas.
Methods
Consecutive patients diagnosed with meningioma by surgery (77 low-grade and 28 non-low-grade meningiomas) underwent preoperative magnetic resonance imaging including T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and contrast-enhanced T1WI (CE-T1WI). Manual segmentation of the tumor area was performed to extract texture features. Segmentation of the peritumoral area was performed for peritumoral high-signal intensity (PHSI) on T2WI. Principal component analysis was performed to fuse the texture features to principal components (PCs), and PCs of each sequence of the tumor and peritumoral areas were compared between low- and non-low-grade meningiomas. Only PCs with statistical significance were used for the model construction using a support vector machine algorithm. k-fold cross-validation with receiver operating characteristic curve analysis was used to evaluate diagnostic performance.
Results
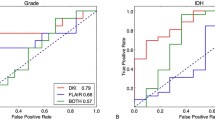
Two, one, and three PCs of T1WI, apparent diffusion coefficient (ADC), and CE-T1WI, respectively, for the tumor area, were significantly different between low- and non-low-grade meningiomas, while PCs of T2WI for the tumor area and PCs for the peritumoral area were not. No significant differences were observed in PHSI. Among models of sequence combination, the model with PCs of ADC and CE-T1WI for the tumor area showed the highest area under the curve (0.84).
Conclusion
The model with PCs of ADC and CE-T1WI for the tumor area showed the highest diagnostic performance for differentiating between low- and non-low-grade meningiomas. Neither PHSI nor PCs in the peritumoral area showed added value.




Similar content being viewed by others
Data availability
No.
Code availability
N/A.
The authors have nothing to disclose.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- CE-T1WI:
-
Contrast-enhanced T1WI
- DWI:
-
Diffusion-weighted imaging
- MRI:
-
Magnetic resonance imaging
- PHSI:
-
Peritumoral high-signal intensity
- PCA:
-
Principal component analysis
- PC:
-
Principal component
- T1WI:
-
T1-weighted imaging
- T2WI:
-
T2-weighted imaging
References
Wiemels J, Wrensch M, Claus EB (2010) Epidemiology and etiology of meningioma. J Neurooncol 99:307–314. https://doi.org/10.1007/s11060-010-0386-3
Wen Patrick Y (2016) World Health Organization Classification of Central Nervous System Tumors. Continuum (Minneap Minn) 23(6):1531–1547
Gritsch S, Batchelor TT, Gonzalez Castro LN (2022) Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer 128:47–58. https://doi.org/10.1002/cncr.33918
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Modha A, Gutin PH (2005) Diagnosis and treatment of atypical and anaplastic meningiomas: a review. Neurosurgery 57:538–550. https://doi.org/10.1227/01.NEU.0000170980.47582.A5
Endo T, Narisawa A, Ali HSM et al (2016) A study of prognostic factors in 45 cases of atypical meningioma. Acta Neurochir 158:1661–1667. https://doi.org/10.1007/s00701-016-2900-7
Sun SQ, Cai C, Murphy RKJ et al (2014) Management of atypical cranial meningiomas, part 2. Neurosurgery 75:356–363. https://doi.org/10.1227/NEU.0000000000000462
Li D, Jiang P, Xu S et al (2019) Survival impacts of extent of resection and adjuvant radiotherapy for the modern management of high-grade meningiomas. J Neurooncol 145:125–134. https://doi.org/10.1007/s11060-019-03278-w
Goldbrunner R, Minniti G, Preusser M et al (2016) EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol 17:e383–e391. https://doi.org/10.1016/S1470-2045(16)30321-7
Spille DC, Sporns PB, Heß K et al (2019) Prediction of high-grade histology and recurrence in meningiomas using routine preoperative magnetic resonance imaging: a systematic review. World Neurosurgery 128:174–181. https://doi.org/10.1016/j.wneu.2019.05.017
Hashiba T, Hashimoto N, Maruno M et al (2006) Scoring radiologic characteristics to predict proliferative potential in meningiomas. Brain Tumor Pathol 23:49–54. https://doi.org/10.1007/s10014-006-0199-4
Hsu C-C, Pai C-Y, Kao H-W et al (2010) Do aggressive imaging features correlate with advanced histopathological grade in meningiomas? J Clin Neurosci 17:584–587. https://doi.org/10.1016/j.jocn.2009.09.018
Ueno Y, Forghani B, Forghani R et al (2017) Endometrial carcinoma: MR imaging–based texture model for preoperative risk stratification—a preliminary analysis. Radiology 284(3):748–757. https://doi.org/10.1148/radiol.2017161950
Yamada I, Miyasaka N, Kobayashi D et al (2019) Endometrial carcinoma: texture analysis of apparent diffusion coefficient maps and its correlation with histopathologic findings and prognosis. Radiology Imaging Cancer 1:e190054. https://doi.org/10.1148/rycan.2019190054
Mori N, Abe H, Mugikura S et al (2021) Discriminating low-grade ductal carcinoma in situ (DCIS) from non-low-grade DCIS or DCIS upgraded to invasive carcinoma: effective texture features on ultrafast dynamic contrast-enhanced magnetic resonance imaging. Breast Cancer 28:1141–1153. https://doi.org/10.1007/s12282-021-01257-6
Ren H, Mori N, Mugikura S et al (2021) Prediction of placenta accreta spectrum using texture analysis on coronal and sagittal T2-weighted imaging. Abdom Radiol 46:5344–5352. https://doi.org/10.1007/s00261-021-03226-1
Haralick RM, Shanmugam K, Dinstein I (1973) Textural features for image classification. IEEE Trans Syst Man Cybern 6:610–621
Chu H, Lin X, He J et al (2020) Value of MRI radiomics based on enhanced T1WI Images in prediction of meningiomas grade. Acad Radiol 28(5):687–693. https://doi.org/10.1016/j.acra.2020.03.034
Yan P-F, Yan L, Hu T-T et al (2017) The potential value of preoperative MRI texture and shape analysis in grading meningiomas: a preliminary investigation. Translational Oncology 10:570–577. https://doi.org/10.1016/j.tranon.2017.04.006
Chen C, Guo X, Wang J et al (2019) The diagnostic value of radiomics-based machine learning in predicting the grade of meningiomas using conventional magnetic resonance imaging: a preliminary study. Front Oncol 9:1338. https://doi.org/10.3389/fonc.2019.01338
Park YW, Oh J, You SC et al (2019) Radiomics and machine learning may accurately predict the grade and histological subtype in meningiomas using conventional and diffusion tensor imaging. Eur Radiol 29:4068–4076. https://doi.org/10.1007/s00330-018-5830-3
Hamerla G, Meyer H-J, Schob S et al (2019) Comparison of machine learning classifiers for differentiation of grade 1 from higher gradings in meningioma: a multicenter radiomics study. Magn Reson Imaging 63:244–249. https://doi.org/10.1016/j.mri.2019.08.011
Laukamp KR, Shakirin G, Baeßler B et al (2019) Accuracy of radiomics-based feature analysis on multiparametric magnetic resonance images for noninvasive meningioma grading. World Neurosurgery 132:e366–e390. https://doi.org/10.1016/j.wneu.2019.08.148
Ugga L, Perillo T, Cuocolo R et al (2021) Meningioma MRI radiomics and machine learning: systematic review, quality score assessment, and meta-analysis. Neuroradiology 63:1293–1304. https://doi.org/10.1007/s00234-021-02668-0
Adeli A, Hess K, Mawrin C et al (2018) Prediction of brain invasion in patients with meningiomas using preoperative magnetic resonance imaging. Oncotarget 9:35974–35982. https://doi.org/10.18632/oncotarget.26313
Amano T, Nakamizo A, Murata H et al (2022) Preoperative prediction of intracranial meningioma grade using conventional CT and MRI. Cureus 25;14(1):e21610. https://doi.org/10.7759/cureus.21610
Shin C, Kim JM, Cheong JH et al (2021) Association between tumor size and peritumoral brain edema in patients with convexity and parasagittal meningiomas. PLoS One 16:e0252945. https://doi.org/10.1371/journal.pone.0252945
Toh CH, Castillo M (2021) Peritumoral brain edema volume in meningioma correlates with tumor fractional anisotropy but not apparent diffusion coefficient or cerebral blood volume. Neuroradiology 63:1263–1270. https://doi.org/10.1007/s00234-021-02646-6
Nioche C, Orlhac F, Boughdad S et al (2018) LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res 78:4786–4789. https://doi.org/10.1158/0008-5472.CAN-18-0125
ACTION consortium, Carreras G, Miccinesi G, et al (2021) Missing not at random in end of life care studies: multiple imputation and sensitivity analysis on data from the ACTION study. BMC Med Res Methodol 21:13. https://doi.org/10.1186/s12874-020-01180-y
Ringnér M (2008) What is principal component analysis? Nat Biotechnol 26:303–304. https://doi.org/10.1038/nbt0308-303
Wang H, Schabath MB, Liu Y et al (2015) Semiquantitative computed tomography characteristics for lung adenocarcinoma and their association with lung cancer survival. Clin Lung Cancer 16:e141–e163. https://doi.org/10.1016/j.cllc.2015.05.007
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159. https://doi.org/10.2307/2529310
Chen C, Qin Y, Cheng J et al (2021) Texture analysis of fat-suppressed T2-weighted magnetic resonance imaging and use of machine learning to discriminate nasal and paranasal sinus small round malignant cell tumors. Front Oncol 11:701289. https://doi.org/10.3389/fonc.2021.701289
Tibshirani R (1996) Regression shrinkage and selection via the lasso. J Roy Stat Soc: Ser B (Methodol) 58:267–288. https://doi.org/10.1111/j.2517-6161.1996.tb02080.x
Corrias G, Micheletti G, Barberini L et al (2022) Texture analysis imaging “what a clinical radiologist needs to know.” Eur J Radiol 146:110055. https://doi.org/10.1016/j.ejrad.2021.110055
Abdi H, Williams LJ (2010) Principal component analysis: principal component analysis. WIREs Comp Stat 2:433–459. https://doi.org/10.1002/wics.101
behzadmehr R, behzadmehr R, (2021) Are the clinical manifestations of CT scan and location associated with World Health Organization histopathological grades of meningioma?: a retrospective study. Annals of Medicine and Surgery 66:102365. https://doi.org/10.1016/j.amsu.2021.102365
Kandemirli SG, Chopra S, Priya S et al (2020) Presurgical detection of brain invasion status in meningiomas based on first-order histogram based texture analysis of contrast enhanced imaging. Clin Neurol Neurosurg 198:106205. https://doi.org/10.1016/j.clineuro.2020.106205
Zhang Y, Chen J-H, Chen T-Y et al (2019) Radiomics approach for prediction of recurrence in skull base meningiomas. Neuroradiology 61:1355–1364. https://doi.org/10.1007/s00234-019-02259-0
Joo L, Park JE, Park SY et al (2021) Extensive peritumoral edema and brain-to-tumor interface MRI features enable prediction of brain invasion in meningioma: development and validation. Neuro Oncol 23:324–333. https://doi.org/10.1093/neuonc/noaa190
Gill CM, Loewenstern J, Rutland JW et al (2021) Peritumoral edema correlates with mutational burden in meningiomas. Neuroradiology 63:73–80. https://doi.org/10.1007/s00234-020-02515-8
Brabec J, Szczepankiewicz F, Lennartsson F et al (2022) Histogram analysis of tensor-valued diffusion MRI in meningiomas: relation to consistency, histological grade and type. NeuroImage Clinical 33:102912. https://doi.org/10.1016/j.nicl.2021.102912
Acknowledgements
The authors thank Mamiko Yamazaki and Akari Akiyama in Tohoku University for their kind support.
Funding
This research was supported by JSPS KAKENHI (22K07763).
Author information
Authors and Affiliations
Contributions
Conceptualization: [Naoko Mori], [Shunji Mugikura], and [Toshiki Endo]; methodology: [Shunji Mugikura] and [Naoko Mori]; formal analysis and investigation: [Hidenori Endo], [Li Li], and [Akira Ito]; writing—original draft preparation: [Naoko Mori] and [Yo Oguma]; writing—review and editing: [Shunji Mugikura] and [Kei Takase]; funding acquisition: [Naoko Mori]; resources: [Toshiki Endo], [Hidenori Endo], [Mika Watanabe], and [Masayuki Kanamori]; and supervision: [Teiji Tominaga] and [Kei Takase].
Corresponding author
Ethics declarations
Ethics approval
Our institutional review board (IRB) approved this retrospective study.
Competing interests
The authors have no conflicts of interest to disclose.
Informed consent
IRB waived the need for obtaining written informed consent.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mori, N., Mugikura, S., Endo, T. et al. Principal component analysis of texture features for grading of meningioma: not effective from the peritumoral area but effective from the tumor area. Neuroradiology 65, 257–274 (2023). https://doi.org/10.1007/s00234-022-03045-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-03045-1