Abstract
Purpose
To investigate the abnormal time-varying local spontaneous brain activity in patients with high myopia (HM) on the basis of the dynamic amplitude of low-frequency fluctuations (dALFF) approach.
Methods
Age and gender matching were performed based on resting-state functional magnetic resonance imaging data from 86 HM patients and 87 healthy controls (HCs). Local spontaneous brain activities were evaluated using the time-varying dALFF method. Support vector machine combined with the radial basis function kernel was used for pattern classification analysis.
Results
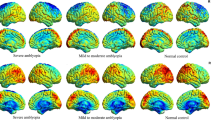
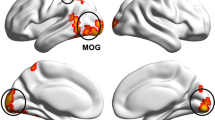
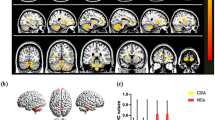
Inter-group comparison between HCs and HM patients has demonstrated that dALFF variability in the left inferior frontal gyrus (orbital part), left lingual gyrus, right anterior cingulate and paracingulate gyri, and right calcarine fissure and surrounding cortex was decreased in HM patients, while increased in the left thalamus, left paracentral lobule, and left inferior parietal (except supramarginal and angular gyri). Pattern classification between HM patients and HCs displayed a classification accuracy of 85.5%.
Conclusion
In this study, the findings mentioned above have suggested the association between local brain activities of HM patients and abnormal variability in brain regions performing visual sensorimotor and attentional control functions. Several useful information has been provided to elucidate the mechanism-related alterations of the myopic nervous system. In addition, the significant role of abnormal dALFF variability has been highlighted to achieve an in-depth comprehension of the pathological alterations and neuroimaging mechanisms in the field of HM.



Similar content being viewed by others
References
Verkicharla PK, Ohno-Matsui K, Saw SM (2015) Current and predicted demographics of high myopia and an update of its associated pathological changes. Ophthalmic Physiol Opt 35:465–475. https://doi.org/10.1111/opo.12238
Ohno-Matsui K, Akiba M, Moriyama M, Ishibashi T, Hirakata A, Tokoro T (2012) Intrachoroidal cavitation in macular area of eyes with pathologic myopia. Am J Ophthalmol 154(2):382–393. https://doi.org/10.1016/j.ajo.2012.02.010
Garcia-Valenzuela E, Kaufman LM (2005) High myopia associated with retinopathy of prematurity is primarily lenticular. J Ame Assoc Pediatr Ophthalmol Strabismus 9(2):121–128. https://doi.org/10.1016/j.jaapos.2004.12.018
Zhu X, Du Y, Li D, Xu J, Wu Q, He W et al (2021) Aberrant TGF-β1 signaling activation by MAF underlies pathological lens growth in high myopia. Nat Commun 12:2102. https://doi.org/10.1038/s41467-021-22041-2
Mirshahi A, Ponto KA, Laubert-Reh D, Rahm B, Lackner KJ, Binder H et al (2016) Myopia and cognitive performance: results from the Gutenberg Health Study. Invest Ophthalmol Vis Sci 57:5230–5236. https://doi.org/10.1167/iovs.16-19507
Tsao W-S, Hsieh H-P, Chuang Y-T, Sheu M-M (2017) Ophthalmologic abnormalities among students with cognitive impairment in eastern Taiwan: the special group with undetected visual impairment. J Formos Med Assoc 116(5):345–350. https://doi.org/10.1016/j.jfma.2016.06.013
Ong S-Y, Ikram MK, Haaland BA, Cheng C-Y, Saw S-M, Wong TY et al (2013) Myopia and cognitive dysfunction: the Singapore Malay Eye Study. Invest Ophthalmol Vis Sci 54:799–803. https://doi.org/10.1167/iovs.12-10460
Huang X, Zhou F, Hu Y, Xu X, Zhou X, Zhong Y et al (2016) Altered spontaneous brain activity pattern in patients with high myopia using amplitude of low-frequency fluctuation: a resting-state fMRI study. Neuropsychiatr Dis Treat 12:2949–2956. https://doi.org/10.2147/ndt.s118326
Zhang X, Dai R, Cheng G, Zhang W, Long Q (2020) Altered amplitude of low-frequency fluctuations and default mode network connectivity in high myopia:a resting-state fMRI study. Int J Ophthalmol 13(10):1629–1636. https://doi.org/10.18240/ijo.2020.10.18
Park B-y, Vos de Wael R, Paquola C, Larivière S, Benkarim O, Royer J et al (2021) Signal diffusion along connectome gradients and inter-hub routing differentially contribute to dynamic human brain function. Neuroimage 224:117429. https://doi.org/10.1016/j.neuroimage.2020.117429
Li R, Liao W, Yu Y, Chen H, Guo X, Tang Y-L et al (2018) Differential patterns of dynamic functional connectivity variability of striato-cortical circuitry in children with benign epilepsy with centrotemporal spikes. Hum Brain Mapp 39(3):1207–1217. https://doi.org/10.1002/hbm.23910
Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD (2014) Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24(3):663–676. https://doi.org/10.1093/cercor/bhs352
Themanson JR, Rosen PJ, Pontifex MB, Hillman CH, McAuley E (2012) Alterations in error-related brain activity and post-error behavior over time. Brain Cogn 80(2):257–265. https://doi.org/10.1016/j.bandc.2012.07.003
Damaraju E, Allen EA, Belger A, Ford JM, McEwen S, Mathalon DH et al (2014) Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage: Clin 5:298–308. https://doi.org/10.1016/j.nicl.2014.07.003
Liao W, Li J, Duan X, Cui Q, Chen H, Chen H (2018) Static and dynamic connectomics differentiate between depressed patients with and without suicidal ideation. Hum Brain Mapp 39:4105–4118. https://doi.org/10.1002/hbm.24235
Pang Y, Chen H, Wang Y, Long Z, He Z, Zhang H et al (2018) Transdiagnostic and diagnosis-specific dynamic functional connectivity anchored in the right anterior insula in major depressive disorder and bipolar depression. Prog Neuropsychopharmacol Biol Psychiatry 85:7–15. https://doi.org/10.1016/j.pnpbp.2018.03.020
Guo X, Duan X, Suckling J, Chen H, Liao W, Cui Q et al (2019) Partially impaired functional connectivity states between right anterior insula and default mode network in autism spectrum disorder. Hum Brain Mapp 40(4):1264–1275. https://doi.org/10.1002/hbm.24447
Karlaftis VM, Giorgio J, Zamboni E, Frangou P, Rideaux R, Ziminski JJ et al (2021) Functional interactions between sensory and memory networks for adaptive behavior. Cereb Cortex 31(12):5319–5330. https://doi.org/10.1093/cercor/bhab160
Sauseng P, Klimesch W (2008) What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci Biobehav Rev 32(5):1001–1013. https://doi.org/10.1016/j.neubiorev.2008.03.014
Jao T, Vértes PE, Alexander-Bloch AF, Tang IN, Yu Y-C, Chen J-H et al (2013) Volitional eyes opening perturbs brain dynamics and functional connectivity regardless of light input. Neuroimage 69:21–34. https://doi.org/10.1016/j.neuroimage.2012.12.007
Liu C-H, Li F, Li S-F, Wang Y-J, Tie C-L, Wu H-Y et al (2012) Abnormal baseline brain activity in bipolar depression: a resting state functional magnetic resonance imaging study. Psychiatry Res: Neuroimaging 203(2):175–179. https://doi.org/10.1016/j.pscychresns.2012.02.007
Zang Y, He Y, Zhu C, Cao Q, Sui M, Liang M et al (2007) Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Develop 29(2):83–91. https://doi.org/10.1016/j.braindev.2006.07.002
Li Q, Huang X, Ye L, Wei R, Zhang Y, Zhong Y-L et al (2016) Altered spontaneous brain activity pattern in patients with late monocular blindness in middle-age using amplitude of low-frequency fluctuation: a resting-state functional MRI study. Clin Interv Aging 11:1773–1780. https://doi.org/10.2147/CIA.S117292
Min Y, Su T, Shu Y, Liu W, Chen L, Shi W et al (2018) Altered spontaneous brain activity patterns in strabismus with amblyopia patients using amplitude of low-frequency fluctuation: a resting-state fMRI study. Neuropsychiatr Dis Treat 14:2351–2359. https://doi.org/10.2147/NDT.S171462
Li T, Liu Z, Li J, Liu Z, Tang Z, Xie X et al (2015) Altered amplitude of low-frequency fluctuation in primary open-angle glaucoma: a resting-state fMRI study. Invest Ophthalmol Vis Sci 56:322–329. https://doi.org/10.1167/iovs.14-14974
Cui Q, Sheng W, Chen Y, Pang Y, Lu F, Tang Q et al (2020) Dynamic changes of amplitude of low-frequency fluctuations in patients with generalized anxiety disorder. Hum Brain Mapp 41(6):1667–1676. https://doi.org/10.1002/hbm.24902
Li J, Duan X, Cui Q, Chen H, Liao W (2019) More than just statics: temporal dynamics of intrinsic brain activity predicts the suicidal ideation in depressed patients. Psychol Med 49(5):852–860. https://doi.org/10.1017/S0033291718001502
Yan C, Wang X, Zuo X, Zang Y (2016) DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics 14:339–351. https://doi.org/10.1007/s12021-016-9299-4
Avants BB, Epstein CL, Grossman M, Gee JC (2008) Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12(1):26–41. https://doi.org/10.1016/j.media.2007.06.004
Sharaev MG, Zavyalova VV, Ushakov VL, Kartashov SI, Velichkovsky BM (2016) Effective connectivity within the default mode network: dynamic causal modeling of resting-state fMRI data. Frontiers in Human Neuroscience 10(14). https://doi.org/10.3389/fnhum.2016.00014
Gratton C, Dworetsky A, Coalson RS, Adeyemo B, Laumann TO, Wig GS et al (2020) Removal of high frequency contamination from motion estimates in single-band fMRI saves data without biasing functional connectivity. Neuroimage 217:116866. https://doi.org/10.1016/j.neuroimage.2020.116866
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2013) Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage 76:439–441. https://doi.org/10.1016/j.neuroimage.2012.03.017
Tomasi D, Shokri-Kojori E, Volkow ND (2016) Temporal changes in local functional connectivity density reflect the temporal variability of the amplitude of low frequency fluctuations in gray matter. PLoS One 11(4):e0154407. https://doi.org/10.1371/journal.pone.0154407
Shen H, Li Z, Zeng L-L, Yuan L, Chen F, Liu Z et al (2014) Internetwork dynamic connectivity effectively differentiates schizophrenic patients from healthy controls. NeuroReport 25(17):1344–1349. https://doi.org/10.1097/wnr.0000000000000267
Hutchison RM, Womelsdorf T, Gati JS, Everling S, Menon RS (2013) Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum Brain Mapp 34(9):2154–2177. https://doi.org/10.1002/hbm.22058
Hindriks R, Adhikari MH, Murayama Y, Ganzetti M, Mantini D, Logothetis NK et al (2016) Can sliding-window correlations reveal dynamic functional connectivity in resting-state fMRI? Neuroimage 127:242–256. https://doi.org/10.1016/j.neuroimage.2015.11.055
Liao W, Li J, Ji GJ, Wu GR, Long Z, Xu Q et al (2019) Endless fluctuations: temporal dynamics of the amplitude of low frequency fluctuations. IEEE Trans Med Imaging 38(11):2523–2532. https://doi.org/10.1109/TMI.2019.2904555
Liao W, Wu G-R, Xu Q, Ji G-J, Zhang Z, Zang Y-F et al (2014) DynamicBC: A MATLAB Toolbox for dynamic brain connectome analysis. Brain Connectivity 4(10):780–790. https://doi.org/10.1089/brain.2014.0253
Burges C (1998) A tutorial on support vector machines for pattern recognition. Data Min Knowl Disc 2:121–167. https://doi.org/10.1023/A:1009715923555
Zhang X, Liu J, Chen Y, Jin Y, Cheng J, for the Alzheimer’s Disease Neuroimaging I (2021) Brain network construction and analysis for patients with mild cognitive impairment and Alzheimer’s disease based on a highly-available nodes approach. Brain and Behav 11:e02027. https://doi.org/10.1002/brb3.2027
Chang C-C, Lin C-J (2011) LIBSVM: a library for support vector machines. ACM Trans Intell Syst Technol 2(3):27. https://doi.org/10.1145/1961189.1961199
Xia M, Wang J, He Y (2013) BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One 8(7):e68910. https://doi.org/10.1371/journal.pone.0068910
Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA et al (2003) Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6(7):750–757. https://doi.org/10.1038/nn1075
Kurata K (2005) Activity properties and location of neurons in the motor thalamus that project to the cortical motor areas in monkeys. J Neurophysiol 94(1):550–566. https://doi.org/10.1152/jn.01034.2004
Karlen SJ, Kahn DM, Krubitzer L (2006) Early blindness results in abnormal corticocortical and thalamocortical connections. Neuroscience 142(3):843–858. https://doi.org/10.1016/j.neuroscience.2006.06.055
Qin W, Yu C (2013) Neural pathways conveying novisual information to the visual cortex. Neural Plasticity 2013.https://doi.org/10.1155/2013/864920
Lin X, Ding K, Liu Y, Yan X, Song S, Jiang T (2012) Altered spontaneous activity in anisometropic amblyopia subjects: revealed by resting-state fMRI. PLoS One 7(8):e43373. https://doi.org/10.1371/journal.pone.0043373
Sathian K, Zangaladze A (2001) Feeling with the mind’s eye: the role of visual imagery in tactile perception. Optom Vis Sci 78(5):276–281. https://doi.org/10.1097/00006324-200105000-00010
Burton H, Sinclair RJ, McLaren DG (2004) Cortical activity to vibrotactile stimulation: an fMRI study in blind and sighted individuals. Hum Brain Mapp 23(4):210–228. https://doi.org/10.1002/hbm.20064
Négyessy L, Nepusz T, Kocsis L, Bazsó F (2006) Prediction of the main cortical areas and connections involved in the tactile function of the visual cortex by network analysis. Eur J Neurosci 23:1919–1930. https://doi.org/10.1111/j.1460-9568.2006.04678.x
Liu Y, Yu C, Liang M, Li J, Tian L, Zhou Y et al (2007) Whole brain functional connectivity in the early blind. Brain 130(8):2085–2096. https://doi.org/10.1093/brain/awm121
Yu C, Liu Y, Li J, Zhou Y, Wang K, Tian L et al (2008) Altered functional connectivity of primary visual cortex in early blindness. Hum Brain Mapp 29:533–543. https://doi.org/10.1002/hbm.20420
Yu C, Shu N, Li J, Qin W, Jiang T, Li K (2007) Plasticity of the corticospinal tract in early blindness revealed by quantitative analysis of fractional anisotropy based on diffusion tensor tractography. Neuroimage 36(2):411–417. https://doi.org/10.1016/j.neuroimage.2007.03.003
Clower DM, West RA, Lynch JC, Strick PL (2001) The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci 21(16):6283–6291. https://doi.org/10.1523/JNEUROSCI.21-16-06283.2001
Wu Y, Wu N, Huang X, Rao J, Yan L, Shi L et al (2020) Evidence of cortical thickness reduction and disconnection in high myopia. Sci Rep 10:16239. https://doi.org/10.1038/s41598-020-73415-3
Sulpizio V, Committeri G, Lambrey S, Berthoz A, Galati G (2013) Selective role of lingual/parahippocampal gyrus and retrosplenial complex in spatial memory across viewpoint changes relative to the environmental reference frame. Behav Brain Res 242:62–75. https://doi.org/10.1016/j.bbr.2012.12.031
Vachon P, Voss P, Lassonde M, Leroux JM, Mensour B, Beaudoin G et al (2013) Reorganization of the auditory, visual and multimodal areas in early deaf individuals. Neurosci 245:50–60. https://doi.org/10.1016/j.neuroscience.2013.04.004
Huang X, Zhou F-Q, Dan H-D, Shen Y (2018) Abnormal intrinsic brain activity in individuals with peripheral vision loss because of retinitis pigmentosa using amplitude of low-frequency fluctuations. NeuroReport 29(15):1323–1332. https://doi.org/10.1097/WNR.0000000000001116
Dan H-D, Zhou F-Q, Huang X, Xing Y-Q, Shen Y (2019) Altered intra- and inter-regional functional connectivity of the visual cortex in individuals with peripheral vision loss due to retinitis pigmentosa. Vision Res 159:68–75. https://doi.org/10.1016/j.visres.2019.02.013
Fornito A, Whittle S, Wood SJ, Velakoulis D, Pantelis C, Yücel M (2006) The influence of sulcal variability on morphometry of the human anterior cingulate and paracingulate cortex. Neuroimage 33(3):843–854. https://doi.org/10.1016/j.neuroimage.2006.06.061
Cheng Y, Yan L, Hu L, Wu H, Huang X, Tian Y et al (2020) Differences in network centrality between high and low myopia: a voxel-level degree centrality study. Acta Radiol 61(10):1388–1397. https://doi.org/10.1177/0284185120902385
Belliveau JW, Kennedy DN, McKinstry RC, Buchbinder BR, Weisskoff RM, Cohen MS et al (1991) Functional mapping of the human visual cortex by magnetic resonance imaging. Science 254(5032):716–719. https://doi.org/10.1126/science.1948051
Ji D, Wilson MA (2007) Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci 10(1):100–107. https://doi.org/10.1038/nn1825
Plailly J, d’Amato T, Saoud M, Royet J-P (2006) Left temporo-limbic and orbital dysfunction in schizophrenia during odor familiarity and hedonicity judgments. Neuroimage 29(1):302–313. https://doi.org/10.1016/j.neuroimage.2005.06.056
Catani M, Dell’Acqua F, Vergani F, Malik F, Hodge H, Roy P et al (2012) Short frontal lobe connections of the human brain. Cortex 48(2):273–291. https://doi.org/10.1016/j.cortex.2011.12.001
Sinding C, Hummel T, Béno N, Prescott J, Bensafi M, Coureaud G et al (2021) Configural memory of a blending aromatic mixture reflected in activation of the left orbital part of the inferior frontal gyrus. Behav Brain Res 402:113088. https://doi.org/10.1016/j.bbr.2020.113088
Wu X, Kong X, Vatansever D, Liu Z, Zhang K, Sahakian BJ et al (2022) Dynamic changes in brain lateralization correlate with human cognitive performance. PLoS Biol 20(3):e3001560. https://doi.org/10.1371/journal.pbio.3001560
Rinaldi L, Ciricugno A, Merabet LB, Vecchi T, Cattaneo Z (2020) The effect of blindness on spatial asymmetries. Brain Sci 10(10):662. https://doi.org/10.3390/brainsci10100662
Moosmann M, Ritter P, Krastel I, Brink A, Thees S, Blankenburg F et al (2003) Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. Neuroimage 20(1):145–158. https://doi.org/10.1016/S1053-8119(03)00344-6
Wang Z, Yan C, Zhao C, Qi Z, Zhou W, Lu J et al (2011) Spatial patterns of intrinsic brain activity in mild cognitive impairment and Alzheimer’s disease: a resting-state functional MRI study. Hum Brain Mapp 32:1720–1740. https://doi.org/10.1002/hbm.21140
Faghiri A, Stephen JM, Wang Y-P, Wilson TW, Calhoun VD (2018) Changing brain connectivity dynamics: from early childhood to adulthood. Hum Brain Mapp 39(3):1108–1117. https://doi.org/10.1002/hbm.23896
Fu Z, Tu Y, Di X, Du Y, Pearlson GD, Turner JA et al (2018) Characterizing dynamic amplitude of low-frequency fluctuation and its relationship with dynamic functional connectivity: an application to schizophrenia. Neuroimage 180:619–631. https://doi.org/10.1016/j.neuroimage.2017.09.035
Funding
This work was supported by The First Affiliated Hospital of Zhengzhou University (Grant No: YNQN2017160) and Henan Province Key R&D and Promotion Project (Science and Technology Research) (Grant No: 222102310317).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest exist.
Ethics approval
This study followed the tenets of the Declaration of Helsinki, and was approved by the First Affiliated Hospital of Zhengzhou University Scientific research and clinical trial ethics committee (No: KY-2021–00659).
Informed consent
Informed consent to participate was obtained from all patients.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Liu, L., Jin, X. et al. Altered time-varying local spontaneous brain activity pattern in patients with high myopia: a dynamic amplitude of low-frequency fluctuations study. Neuroradiology 65, 157–166 (2023). https://doi.org/10.1007/s00234-022-03033-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-03033-5