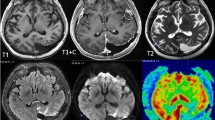
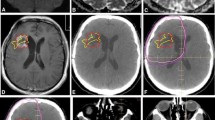
Abstract
Purpose
Previous diffusion tensor imaging (DTI) studies have mainly focused on dose-dependent white matter (WM) alterations 1 month to 1 year after radiation therapy (RT) with a tract-average method. However, WM alterations immediately after RT are subtle, resulting in early WM alterations that cannot be detected by tract-average methods. Therefore, we performed a study with an along-tract method in patients with brain metastases to explore the early dose–response pattern of WM alterations after RT.
Methods
Sixteen patients with brain metastases underwent DTI before and 1–3 days after brain RT. DTI metrics, such as fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD) and mean diffusivity (MD), were calculated. Along-tract statistics were then used to resample WM fibre streamlines and generate a WM skeleton fibre tract. DTI metric alterations (post_RT-pre_RT DTI metrics) and the planned doses (max or mean doses) were mapped to 18 WM tracts. A linear fixed model was performed to analyse the main effect of dose on DTI metric alterations.
Results
AD alterations in the left hemispheric uncinated fasciculus (UNC_L) were associated with max doses, in which decreased AD alterations were associated with higher doses.
Conclusion
Our findings may provide pathological insight into early dose-dependent WM alterations and may contribute to the development of max dose-constrained RT techniques to protect brain microstructure in the UNC_L.



Similar content being viewed by others
Abbreviations
- AD:
-
Axial diffusivity
- CG:
-
Cinguli
- CNS:
-
Central nervous system
- CST:
-
Corticospinal tract
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- FACT:
-
Fibre assignment by continuous
- FLAIR:
-
Fluid-attenuated inversion recovery
- IFO:
-
Inferior fronto-occipital tract
- ILF:
-
Inferior longitudinal fasciculus
- MD:
-
Mean diffusivity
- MRI:
-
Magnetic resonance imaging
- RD:
-
Radial diffusivity
- RT:
-
Radiation therapy
- SLF-fp:
-
Superior longitudinal fasciculus anterior segment
- SLF-pt:
-
Superior longitudinal fasciculus posterior segment
- SLF-t:
-
Superior longitudinal fasciculus long segment
- UNC:
-
Uncinated fasciculus
- WM:
-
White matter
References
Suh JH, Kotecha R, Chao ST, Ahluwalia MS, Sahgal A, Chang EL (2020) Current approaches to the management of brain metastases. Nat Rev Clin Oncol 17:279–299
Olson R, Tyldesley S, Carolan H, Parkinson M, Chhanabhai T, McKenzie M (2011) Prospective comparison of the prognostic utility of the Mini Mental State Examination and the Montreal Cognitive Assessment in patients with brain metastases. Support Care Cancer 19:1849–1855
Li J, Bentzen SM, Li J, Renschler M, Mehta MP (2008) Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys 71:64–70
Mahajan A, Ahmed S, McAleer MF et al (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1040–1048
Brown PD, Jaeckle K, Ballman KV et al (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA 316:401–409
Fan L (2021) Mapping the human brain: what is the next frontier? Innovation (N Y) 2:100073
Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT (2005) Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res 164:662–668
Tsuruda JS, Kortman KE, Bradley WG, Wheeler DC, Van Dalsem W, Bradley TP (1987) Radiation effects on cerebral white matter: MR evaluation. AJR Am J Roentgenol 149:165–171
Lee WH, Sonntag WE, Mitschelen M, Yan H, Lee YW (2010) Irradiation induces regionally specific alterations in pro-inflammatory environments in rat brain. Int J Radiat Biol 86:132–144
Warrington JP, Ashpole N, Csiszar A, Lee YW, Ungvari Z, Sonntag WE (2013) Whole brain radiation-induced vascular cognitive impairment: mechanisms and implications. J Vasc Res 50:445–457
Ljubimova NV, Levitman MK, Plotnikova ED, Eidus L (1991) Endothelial cell population dynamics in rat brain after local irradiation. Br J Radiol 64:934–940
Wu PH, Coultrap S, Pinnix C et al (2012) Radiation induces acute alterations in neuronal function. PLoS ONE 7:e37677
Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD (2012) Radiation-induced brain injury: a review. Front Oncol 2:73
Makale MT, McDonald CR, Hattangadi-Gluth JA, Kesari S (2017) Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol 13:52–64
Cramer CK, Cummings TL, Andrews RN et al (2019) Treatment of radiation-induced cognitive decline in adult brain tumor patients. Curr Treat Options Oncol 20:42
Tournier JD (2019) Diffusion MRI in the brain - theory and concepts. Prog Nucl Magn Reson Spectrosc 112–113:1–16
Zhu T, Chapman CH, Tsien C et al (2016) Effect of the maximum dose on white matter fiber bundles using longitudinal diffusion tensor imaging. Int J Radiat Oncol Biol Phys 96:696–705
Connor M, Karunamuni R, McDonald C et al (2017) Regional susceptibility to dose-dependent white matter damage after brain radiotherapy. Radiother Oncol 123:209–217
Raschke F, Wesemann T, Wahl H et al (2019) Reduced diffusion in normal appearing white matter of glioma patients following radio (chemo)therapy. Radiother Oncol 140:110–115
Hope TR, Vardal J, Bjornerud A et al (2015) Serial diffusion tensor imaging for early detection of radiation-induced injuries to normal-appearing white matter in high-grade glioma patients. J Magn Reson Imaging 41:414–423
Chapman CH, Nagesh V, Sundgren PC et al (2012) Diffusion tensor imaging of normal-appearing white matter as biomarker for radiation-induced late delayed cognitive decline. Int J Radiat Oncol Biol Phys 82:2033–2040
Connor M, Karunamuni R, McDonald C et al (2016) Dose-dependent white matter damage after brain radiotherapy. Radiother Oncol 121:209–216
Colby JB, Soderberg L, Lebel C, Dinov ID, Thompson PM, Sowell ER (2012) Along-tract statistics allow for enhanced tractography analysis. Neuroimage 59:3227–3242
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Cox RW (2012) AFNI: what a long strange trip it’s been. Neuroimage 62:743–747
Zhang Y, Zhang J, Oishi K et al (2010) Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage 52:1289–1301
Oishi K, Faria A, Jiang H et al (2009) Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participants. Neuroimage 46:486–499
Mori S, Crain BJ, Chacko VP, van Zijl PC (1999) Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45:265–269
Corouge I, Gouttard S, Gerig G (2004) Towards a shape model of white matter fiber bundles using diffusion tensor MRI2004 2nd IEEE International Symposium on Biomedical Imaging: Nano to Macro (IEEE Cat No 04EX821), pp 344–347 Vol. 341
Pinheiro JC, Bates DM (1996) Unconstrained parametrizations for variance-covariance matrices. Stat Comput 6:289–296
Parihar VK, Pasha J, Tran KK, Craver BM, Acharya MM, Limoli CL (2015) Persistent changes in neuronal structure and synaptic plasticity caused by proton irradiation. Brain Struct Funct 220:1161–1171
Gondi V, Pugh SL, Tome WA et al (2014) Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 32:3810–3816
Gondi V, Pugh S, Brown PD et al (2018) NCOG-01. Preservation of neurocognitive function (NCF) with hippocampal avoidance during whole-brain radiotherapy (WBRT) For brain metastases: preliminary results of phase iii trial nrg oncology CC001. Neuro-Oncology 20:vi172
Follin C, Svärd D, van Westen D et al (2019) Microstructural white matter alterations associated to neurocognitive deficits in childhood leukemia survivors treated with cranial radiotherapy - a diffusional kurtosis study. Acta Oncol 58:1021–1028
Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR (2013) Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain 136:1692–1707
Hein TC, Mattson WI, Dotterer HL et al (2018) Amygdala habituation and uncinate fasciculus connectivity in adolescence: a multi-modal approach. Neuroimage 183:617–626
Wang HZ, Qiu SJ, Lv XF et al (2012) Diffusion tensor imaging and 1H-MRS study on radiation-induced brain injury after nasopharyngeal carcinoma radiotherapy. Clin Radiol 67:340–345
Hwang SY, Jung JS, Kim TH et al (2006) Ionizing radiation induces astrocyte gliosis through microglia activation. Neurobiol Dis 21:457–467
Kurita H, Kawahara N, Asai A, Ueki K, Shin M, Kirino T (2001) Radiation-induced apoptosis of oligodendrocytes in the adult rat brain. Neurol Res 23:869–874
Witzmann K, Raschke F, Troost EGC (2021) MR image changes of normal-appearing brain tissue after radiotherapy. Cancers (Basel) 13:1573
Acknowledgements
We thank the Hefei Cancer hospital, Chinese Academy of Science, for their help and support in this project.
Funding
This work was supported by the Key R&D Program of Anhui Province (grant numbers:201904a07020104), the Natural Science Fund of Anhui Province (grant numbers:2008085MC69), Collaborative Innovation Program of Hefei Science Center (grant numbers:2020HSC-CIP001, 2021HSC-CIP013), the General scientific research project of Anhui Provincial Health Commission (grant numbers: AHWJ2021b150), the Natural Science Fund of Hefei City (grant numbers:2021033), CAS Anhui Province Key Laboratory of Medical Physics and Technology (grant numbers: LMPT201904) and Director’s Fund of Hefei Cancer Hospital of CAS (grant numbers: YZJJ2019C14, YZJJ2019A04).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
All study participants provided written informed consent. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Hefei Cancer hospital, Chinese academy of science (protocol code SL-KY2020-001 and date of approval,20 January 2020).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Liu, J., Lang, J. et al. Dose-dependent early white matter alterations in patients with brain metastases after radiotherapy. Neuroradiology 65, 167–176 (2023). https://doi.org/10.1007/s00234-022-03020-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-03020-w