Abstract
Purpose
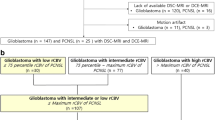
The aim of the study was to compare the parameters of blood flow in glioblastomas and primary central nervous system lymphomas (PCNSLs), measured by pseudo-continuous arterial spin labeling MRI (3D PCASL), and to determine the informativeness of this method in the differential diagnosis between these lesions.
Methods
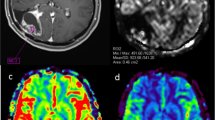
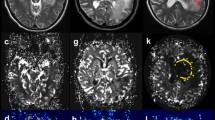
The study included MRI data of 139 patients with PCNSL (n = 21) and glioblastomas (n = 118), performed in the Burdenko Neurosurgical Center. No patients received chemotherapy, hormone therapy, or radiation therapy prior to MRI. On the 3D PCASL perfusion map, the absolute and normalized values of tumor blood flow were calculated in the glioblastoma and PCNSL groups (maxTBFmean and nTBF).
Results
MaxTBFmean and nTBF in the glioblastoma group were significantly higher than those in the PCNSL group: 168.9 ml/100 g/min versus 65.6 and 9.3 versus 3.7, respectively (p < 0.001). Arterial spin labeling perfusion had high sensitivity (86% for maxTBFmean, 95% for nTBF) and specificity (77% for maxTBFmean, 73% for nTBF) in the differential diagnosis between PCNSL and glioblastomas. Blood flow thresholds were 98.9 ml/100 g/min using absolute blood flow values and 6.1 using normalized values, AUC > 0.88.
Conclusion
The inclusion of 3D PCASL in the standard MRI protocol can increase the specificity of the differential diagnosis between glioblastomas and PCNSL.



Similar content being viewed by others
Data availability
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the fact that they could contain sensitive information about patients.
Code availability
The post-processing of the obtained data was carried out using the ReadyView software package (GE Healthcare).
References
Calli C, Kitis O, Yunten N, Yurtseven T, Islekel S, Akalin T (2006) Perfusion and diffusion MR imaging in enhancing malignant cerebral tumors. Eur J Radiol 58:394–403
Ma JH, Kim HS, Rim NJ, Kim SH, Cho KG (2010) Differentiation among glioblastoma multiforme, solitary metastatic tumor, and lymphoma using whole-tumor histogram analysis of the normalized cerebral blood volume in enhancing and perienhancing lesions. AJNR Am J Neuroradiol 31:1699–1706
Alifieris C, Trafalis DT (2015) Glioblastoma multiforme: pathogenesis and treatment. Pharmacol Ther 152:63–82. https://doi.org/10.1016/j.pharmthera.2015.05.005
Bataille B, Delwail V, Menet E et al (2000) Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg 92:261–266
DeAngelis LM (1999) Primary CNS lymphoma: treatment with combined chemotherapy and radiotherapy. J Neurooncol 43:249–257
Labak CM, Holdhoff M, Bettegowda C et al (2019) Surgical resection for primary central nervous system lymphoma: a systematic review. World Neurosurg 126:e1436–e1448. https://doi.org/10.1016/j.wneu.2019.02.252
Hoang Xuan K, Bessell E, Bromberg J, Hottinger AF, Preusser M, Rudà R, Schlegel U, Siegal T, Soussain C, Abacioglu U et al (2015) European Association for Neuro Oncology Task Force on Primary CNS Lymphoma: Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: guidelines from the European Association for Neuro-oncology. Lancet Oncol 16:e322–e332
Ferreri AJ, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, Cabras MG, Fabbri A, Corazzelli G, Ilariucci F et al (2009) International Extranodal Lymphoma Study Group (IELSG): High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet 374:1512–1520
Schlegel U (2009) Primary CNS lymphoma. Ther Adv Neurol Disord 2(2):93–104. https://doi.org/10.1177/1756285608101222
Malikova H, Koubska E, Weichet J et al (2016) Can morphological MRI differentiate between primary central nervous system lymphoma and glioblastoma? Cancer Imaging 16:40
Naka X, Lee M, Buck O et al (2017) Diagnostic accuracy of T1-weighted dynamic contrast-enhanced-MRI and DWI-ADC for differentiation of glioblastoma and primary CNS lymphoma. AJNR Am J Neuroradiol 38:485–491
Guo AC, Cummings TJ, Dash RC, Provenzale JM (2002) Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology 224:177–183
Doskaliyev A, Yamasaki F, Ohtaki M et al (2012) Lymphomas and glioblastomas: differences in the apparent diffusion coefficient evaluated with high b-value diffusion-weighted magnetic resonance imaging at 3T. Eur J Radiol 81:339–344
Wang S, Kim S, Chawla S et al (2011) Differentiation between glioblastomas, solitary brain metastases, and primary cerebral lymphomas using diffusion tensor and dynamic susceptibility contrast-enhanced MR imaging. AJNR Am J Neuroradiol 32:507–514
Toh CH, Castillo M, Wong AM et al (2008) Primary cerebral lymphoma and glioblastoma multiforme: differences in diffusion characteristics evaluated with diffusion tensor imaging. AJNR Am J Neuroradiol 29:471–475
Kinoshita M, Hashimoto N, Goto T et al (2008) Fractional anisotropy and tumor cell density of the tumor core show positive correlation in diffusion tensor magnetic resonance imaging of malignant brain tumors. Neuroimage 43:29–35
Beppu T, Inoue T, Shibata Y et al (2003) Measurement of fractional anisotropy using diffusion tensor MRI in supratentorial astrocytic tumors. J Neurooncol 63:109–116
Toh CH, Wei KC, Chang CN, Ng SH, Wong HF (2013) Differentiation of primary central nervous system lymphomas and glioblastomas: comparisons of diagnostic performance of dynamic susceptibility contrast-enhanced perfusion MR imaging without and with contrast-leakage correction. AJNR Am J Neuroradiol 34:1145–1149
Liao W, Liu Y, Wang X et al (2009) Differentiation of primary central nervous system lymphoma and high-grade glioma with dynamic susceptibility contrast-enhanced perfusion magnetic resonance imaging. Acta Radiol 50:217–225
Murayama K, Nishiyama Y, Hirose Y et al (2018) Differentiating between central nervous system lymphoma and high-grade glioma using dynamic susceptibility contrast and dynamic contrast-enhanced MR imaging with histogram analysis. Magn Reson Med Sci 17:42–49
Choi YS, Lee HJ, Ahn SS et al (2017) Primary central nervous system lymphoma and atypical glioblastoma: differentiation using the initial area under the curve derived from dynamic contrast-enhanced MR and the apparent diffusion coefficient. Eur Radiol 27:1344–1351
Batalov AI, Zakharova NE, Pogosbekyan EL et al (2018) Beskontrastnaia ASL-perfuziia v predoperatsionnoĭ diagnostike supratentorial’nykh gliom [Non-contrast ASL perfusion in preoperative diagnosis of supratentorial gliomas]. Zh Vopr Neirokhir Im N N Burdenko 82(6):15–22. https://doi.org/10.17116/neiro20188206115
Lee JY, Bjørnerud A, Park JE, Lee BE, Kim JH, Kim HS (2019) Permeability measurement using dynamic susceptibility contrast magnetic resonance imaging enhances differential diagnosis of primary central nervous system lymphoma from glioblastoma. Eur Radiol 29(10):5539–5548. https://doi.org/10.1007/s00330-019-06097-9
Lee I et al (2010) Analysis of perfusion weighted image of CNS lymphoma. Eur J Radiol 76:48–51
Hartmann M et al (2003) Distinguishing of primary cerebral lymphoma from HGG with perfusion-weighted MRI. Neurosci Lett 338:119–122
Yamashita K, Yoshiura T, Hiwatashi A et al (2013) Differentiating primary CNS lymphoma from glioblastoma multiforme: assessment using arterial spin labeling, diffusion-weighted imaging, and 18F-fluorodeoxyglucose positron emission tomography. Neuroradiology 55(2):135–143. https://doi.org/10.1007/s00234-012-1089-6
Nakajima S, Okada T, Yamamoto A et al (2015) Differentiation between primary central nervous system lymphoma and glioblastoma: a comparative study of parameters derived from dynamic susceptibility contrast-enhanced perfusion-weighted MRI. Clin Radiol 70:1393–1399
Hakyemez B, Erdogan C, Bolca N, Yildirim N, Gokalp G, Parlak M (2006) Evaluation of different cerebral mass lesions by perfusion weighted MR imaging. J Magn Reson Imaging 24:817–824
Yamashita K, Hiwatashi A, Togao O et al (2016) Diagnostic utility of intravoxel incoherent motion mr imaging in differentiating primary central nervous system lymphoma from glioblastoma multiforme. J Magn Reson Imaging 44:1256–1261
Kickingereder P, Sahm F, Wiestler B et al (2014) Evaluation of microvascular permeability with dynamic contrast-enhanced MRI for the differentiation of primary CNS lymphoma and glioblastoma: radiologic-pathologic correlation. AJNR Am J Neuroradiol 35:1503–1508
Yoo RE, Choi SH, Cho HR, Kim TM, Lee SH, Park C-K, Park S, Kim IH, Yun TJ, Kim JH, Sohn C, Han MH, Chang KH (2013) Tumor blood flow from arterial spin labeling perfusion MRI: a key parameter in distinguishing high-grade gliomas from primary cerebral lymphomas, and in predicting genetic biomarkers in high-grade gliomas. J Magn Reson Imaging 38(4):852–860. https://doi.org/10.1002/jmri.24026
You S, Yun TJ, Choi HJ et al (2018) Differentiation between primary CNS lymphoma and glioblastoma: qualitative and quantitative analysis using arterial spin labeling MR imaging. Eur Radiol 28:3801–3810. https://doi.org/10.1007/s00330-018-5359-5
Rau M, Braun C, Skardelly M, Schittenhelm J, Paulsen F, Bender B, Ernemann U, Bisdas S (2014) Prognostic value of blood flow estimated by arterial spin labeling and dynamic susceptibility contrast-enhanced MR imaging in high grade gliomas. J Neurooncol 120(3):557–566. https://doi.org/10.1007/s11060-014-1586-z
Jiang J, Zhao L, Zhang Y, Zhang S, Yao Y, Qin Y, Wang CY, Zhu W (2014) Comparative analysis of arterial spin labeling and dynamic susceptibility contrast perfusion imaging for quantitative perfusion measurements of brain tumors. Int J Clin Exp Pathol 7(6):2790–2799
Qiu D, Straka M, Zun Z, Bammer R, Moseley M, Zaharchuk G (2012) CBF measurements using multidelay pseudocontinuous and velocity-selective arterial spin labeling in patients with long arterial transit delays: comparison with xenon CT CBF. J Magn Reson Imaging 36(1):110–119. https://doi.org/10.1002/jmri.23613
Ma H, Wang Z, Xu K, Shao Z, Yang C, Xu P, Liu X, Hu C, Lu X, Rong Y (2017) Three-dimensional arterial spin labeling imaging and dynamic susceptibility contrast perfusion-weighted imaging value in diagnosing glioma grade prior to surgery. Exp Ther Med 13(6):2691–2698. https://doi.org/10.3892/etm.2017.4370
Xiao H, Chen Z, Lou X, Wang Y, Gui Q, Wang Y, Shi K, Zhou Z, Zheng D, Wang D, Ma L (2015) Astrocytic tumour grading: a comparative study of three-dimensional pseudocontinuous arterial spin labelling, dynamic susceptibility contrast-enhanced perfusion-weighted imaging, and diffusion-weighted imaging. Eur Radiol 25(12):3423–3430. https://doi.org/10.1007/s00330-015-3768-2
Di N, Cheng W, Chen H, Zhai F, Liu Y, Mu X, Chu Z, Lu N, Liu X, Wang B (2019) Utility of arterial spin labelling MRI for discriminating atypical high-grade glioma from primary central nervous system lymphoma. Clin Radiol 74(2):165.e1-165.e9. https://doi.org/10.1016/j.crad.2018.10.003
Xi YB, Kang XW, Wang N, Liu TT, Zhu YQ, Cheng G, Wang K, Li C, Guo F, Yin H (2019) Differentiation of primary central nervous system lymphoma from high-grade glioma and brain metastasis using arterial spin labeling and dynamic contrast-enhanced magnetic resonance imaging. Eur J Radiol 112:59–64. https://doi.org/10.1016/j.ejrad.2019.01.008
Hatakeyama J, Ono T, Takahashi M, Oda M, Shimizu H (2021) Differentiating between primary central nervous system lymphoma and glioblastoma: the diagnostic value of combining 18F-fluorodeoxyglucose positron emission tomography with arterial spin labeling. Neurol Med Chir (Tokyo). 61(6):367–375. https://doi.org/10.2176/nmc.oa.2020-0375
Funding
This study was supported by the Ministry of Higher Education Agreement 075-15-2021-1343.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of N. N. Burdenko National Medical Research Center of Neurosurgery (protocol code 09/2021, date of approval 10 September 2021).
Consent to participate
Informed consent was obtained from all subjects involved in the study.
Consent for publication
All patients were informed that data could be used in publications.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Batalov, A.I., Afandiev, R.M., Zakharova, N.E. et al. 3D pseudo-continuous arterial spin labeling-MRI (3D PCASL-MRI) in the differential diagnosis between glioblastomas and primary central nervous system lymphomas. Neuroradiology 64, 1539–1545 (2022). https://doi.org/10.1007/s00234-021-02888-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02888-4