Abstract
Purpose
We aimed to analyze the characteristics of brain function and microstructure linked to epilepsy relapse after drug withdrawal in patients with focal epilepsy.
Methods
Resting-state functional magnetic resonance imaging and high-resolution T1-weighted images were acquired within 1 month prior to drug withdrawal from 15 patients who did not have epilepsy relapse (PER − group) and 16 patients who subsequently had epilepsy relapse (PER + group). Additionally, 23 healthy participants undergoing the same scanning protocol were included as controls. Fractional amplitude of low-frequency fluctuation (fALFF) and gray matter density (GMD) were compared among groups. Subgroup and correlation analyses were also performed.
Results
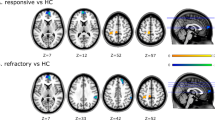
There were no significant differences in fALFF between patient groups, but the PER + group showed lower GMD in the bilateral calcarine, left precuneus, and right superior temporal gyrus than the PER − group (Gaussian random field correction, voxel-level P < 0.001 and cluster-level P < 0.05). Both increased seizure number and polytherapy were associated with lower GMD; also, patients using other antiseizure medications showed lower GMD than those using only levetiracetam (Gaussian random field correction, voxel-level P < 0.001, and cluster-level P < 0.05). The active period and disease duration showed both positive and negative correlations with GMD, while the seizure-free period mainly showed positive correlations with GMD (uncorrected, P < 0.001).
Conclusion
Gray matter microstructure, but not local functional activity, showed distinct characteristics between patients with and without epilepsy relapse and may serve as a potential biomarker for predicting seizure recurrence upon drug withdrawal.




Similar content being viewed by others
Data availability
Data are available upon reasonable request.
Code availability
Not applicable.
References
Kwan P, Brodie MJ (2000) Early identification of refractory epilepsy. N Engl J Med 342:314–319
Perucca P, Gilliam FG (2012) Adverse effects of antiepileptic drugs. Lancet Neurol 11:792–802
Neurology. QSSotAAo, (1996) Practice parameter: a guideline for discontinuing antiepileptic drugs in seizure-free patients–summary statement. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 47:600–602
Lamberink HJ, Otte WM, Geerts AT et al (2017) Individualised prediction model of seizure recurrence and long-term outcomes after withdrawal of antiepileptic drugs in seizure-free patients: a systematic review and individual participant data meta-analysis. Lancet Neurol 16:523–531
Lamberink HJ, Otte WM, Geleijns K et al (2015) Antiepileptic drug withdrawal in medically and surgically treated patients: a meta-analysis of seizure recurrence and systematic review of its predictors. Epileptic Disord 17:211–228
Lin J, Ding S, Li X et al (2020) External validation and comparison of two prediction models for seizure recurrence after the withdrawal of antiepileptic drugs in adult patients. Epilepsia 61:115–124
Gupta L, Janssens R, Vlooswijk MC et al (2017) Towards prognostic biomarkers from BOLD fluctuations to differentiate a first epileptic seizure from new-onset epilepsy. Epilepsia 58:476–483
Yan Y, Xie G, Zhou H et al (2020) Altered spontaneous brain activity in patients with childhood absence epilepsy: associations with treatment effects. Neuroreport 31:613–618
Chen Z, An Y, Zhao B et al (2017) The value of resting-state functional magnetic resonance imaging for detecting epileptogenic zones in patients with focal epilepsy. PLoS One 12:e0172094
Bilevicius E, Yasuda CL, Silva MS et al (2010) Antiepileptic drug response in temporal lobe epilepsy: a clinical and MRI morphometry study. Neurology 75:1695–1701
Keller SS, Cresswell P, Denby C et al (2007) Persistent seizures following left temporal lobe surgery are associated with posterior and bilateral structural and functional brain abnormalities. Epilepsy Res 74:131–139
Wang Z, Zhang Z, Liao W et al (2014) Frequency-dependent amplitude alterations of resting-state spontaneous fluctuations in idiopathic generalized epilepsy. Epilepsy Res 108:853–860
Zeng H, Ramos CG, Nair VA et al (2015) Regional homogeneity (ReHo) changes in new onset versus chronic benign epilepsy of childhood with centrotemporal spikes (BECTS): a resting state fMRI study. Epilepsy Res 116:79–85
Deng DM, Chen LZ, Li YW et al (2019) Cortical morphologic changes in recent-onset, drug-naïve idiopathic generalized epilepsy. Magn Reson Imaging 61:137–142
Perani S, Tierney TM, Centeno M et al (2018) Thalamic volume reduction in drug-naive patients with new-onset genetic generalized epilepsy. Epilepsia 59:226–234
Zhu Y, Yu Y, Shinkareva SV et al (2015) Intrinsic brain activity as a diagnostic biomarker in children with benign epilepsy with centrotemporal spikes. Hum Brain Mapp 36:3878–3889
Zhang Z, Lu G, Zhong Y et al (2010) fMRI study of mesial temporal lobe epilepsy using amplitude of low-frequency fluctuation analysis. Hum Brain Mapp 31:1851–1861
Coan AC, Appenzeller S, Bonilha L et al (2009) Seizure frequency and lateralization affect progression of atrophy in temporal lobe epilepsy. Neurology 73:834–842
Coan AC, Campos BM, Yasuda CL et al (2014) Frequent seizures are associated with a network of gray matter atrophy in temporal lobe epilepsy with or without hippocampal sclerosis. PLoS One 9:e85843
Xiao F, Caciagli L, Wandschneider B et al (2018) Effects of carbamazepine and lamotrigine on functional magnetic resonance imaging cognitive networks. Epilepsia 59:1362–1371
Li X, Ricci R, Large CH et al (2010) Interleaved transcranial magnetic stimulation and fMRI suggests that lamotrigine and valproic acid have different effects on corticolimbic activity. Psychopharmacology 209:233–244
Jokeit H, Okujava M, Woermann FG (2001) Carbamazepine reduces memory induced activation of mesial temporal lobe structures: a pharmacological fMRI-study. BMC Neurol 1:6
Cheng L, Lei S, Chen SH et al (2015) Pretreatment with intravenous levetiracetam in the rhesus monkey Coriaria lactone-induced status epilepticus model. J Neurol Sci 348:111–120
Pardoe HR, Berg AT, Jackson GD (2013) Sodium valproate use is associated with reduced parietal lobe thickness and brain volume. Neurology 80:1895–1900
Scheffer IE, Berkovic S, Capovilla G et al (2017) ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 58:512–521
Fisher RS, Cross JH, French JA et al (2017) Operational classification of seizure types by the International League Against Epilepsy: position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 58:522–530
Tan G, Xiao F, Chen S et al (2018) Frequency-specific alterations in the amplitude and synchronization of resting-state spontaneous low-frequency oscillations in benign childhood epilepsy with centrotemporal spikes. Epilepsy Res 145:178–184
Zeng H, Pizarro R, Nair VA et al (2013) Alterations in regional homogeneity of resting-state brain activity in mesial temporal lobe epilepsy. Epilepsia 54:658–666
Mankinen K, Long XY, Paakki JJ et al (2011) Alterations in regional homogeneity of baseline brain activity in pediatric temporal lobe epilepsy. Brain Res 1373:221–229
van Veenendaal TM, IJff DM, Aldenkamp AP et al (2015) Metabolic and functional MR biomarkers of antiepileptic drug effectiveness: a review. Neurosci Biobehav Rev 59:92–99
Zhang Q, Yang F, Hu Z et al (2017) Resting-state fMRI revealed different brain activities responding to valproic acid and levetiracetam in benign epilepsy with central-temporal spikes. Eur Radiol 27:2137–2145
Park KM, Kim SE, Lee BI et al (2018) Brain morphology in patients with genetic generalized epilepsy: its heterogeneity among subsyndromes. Eur Neurol 80:236–244
Lin JJ, Dabbs K, Riley JD et al (2014) Neurodevelopment in new-onset juvenile myoclonic epilepsy over the first 2 years. Ann Neurol 76:660–668
Hong SJ, Bernhardt BC, Schrader DS et al (2016) Whole-brain MRI phenotyping in dysplasia-related frontal lobe epilepsy. Neurology 86:643–650
Qiu T, Zhang Y, Tang X et al (2019) Precentral degeneration and cerebellar compensation in amyotrophic lateral sclerosis: a multimodal MRI analysis. Hum Brain Mapp 40:3464–3474
Li S, Huang X, Li L et al (2016) Posttraumatic stress disorder: structural characterization with 3-T MR imaging. Radiology 280:537–544
Alhusaini S, Ronan L, Scanlon C et al (2013) Regional increase of cerebral cortex thickness in juvenile myoclonic epilepsy. Epilepsia 54:e138-141
Matricardi S, Operto FF, Farello G et al (2020) Withdrawal seizures: possible risk factors. Expert Rev Neurother 20:667–672
Hsin YL, Harnod T, Chang CS et al (2017) Increase in gray matter volume and white matter fractional anisotropy in the motor pathways of patients with secondarily generalized neocortical seizures. Epilepsy Res 137:61–68
Xu H, Zhu H, Luo L et al (2020) Altered gray matter volume in MRI-negative focal to bilateral tonic-clonic seizures. Acta Neurol Belg. https://doi.org/10.1007/s13760-020-01383-6
Galovic M, van Dooren VQH, Postma TS et al (2019) Progressive cortical thinning in patients with focal epilepsy. JAMA Neurol 76:1230–1239
Wang X, He R, Zheng R et al (2019) Relative seizure relapse risks associated with antiepileptic drug withdrawal after different seizure-free periods in adults with focal epilepsy: a prospective, controlled follow-up study. CNS Drugs 33:1121–1132
Gennatas ED, Avants BB, Wolf DH et al (2017) Age-related effects and sex differences in gray matter density, volume, mass, and cortical thickness from childhood to young adulthood. J Neurosci 37:5065–5073
Acknowledgements
The authors thank all participants for their collaboration.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81420108014), the Post-Doctor Research Project, Sichuan University (Grant No. 20826041E4076), and the Post-Doctor Research Project, West China Hospital, Sichuan University (Grant No. 20HXBH031).
Author information
Authors and Affiliations
Contributions
G.T., L.L. and Q.G. formulated the study design. G.T. and X.L. performed the MRI scanning and image preprocessing. G.T. and D.C. performed statistical analysis and results interpretation. T.G. and L.L. drafted the paper. G.T., L.L. and Q.G revised the paper. G.T. and H.W. recruited participants and collected clinical materials.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflicts of interest.
Ethical approval
This project was approved by the ethical committee of West China Hospital of Sichuan University, and all procedures performed in the study involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Consent for publication
All authors have read and approved the submitted manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tan, G., Li, X., Chen, D. et al. Brain functional and structural characteristics of patients with seizure recurrence following drug withdrawal. Neuroradiology 63, 2087–2097 (2021). https://doi.org/10.1007/s00234-021-02755-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02755-2