Abstract
Purpose
The lifetime risk of developing amyotrophic lateral sclerosis (ALS) increases in the elderly, and greater age at symptom onset has been identified as a negative prognostic factor in the disease. However, the underlying neurobiological mechanisms are still poorly investigated. We hypothesized that older age at symptom onset would have been associated with greater extra-motor cortical damage contributing to worse prognosis, so we explored the relationship between age at symptom onset, cortical thinning (CT) distribution, and clinical markers of disease progression.
Methods
We included 26 ALS patients and 29 healthy controls with T1-weighted magnetic resonance imaging (MRI). FreeSurfer 6.0 was used to identify regions of cortical atrophy (CA) in ALS, and to relate age at symptom onset to CT distribution. Linear regression analyses were then used to investigate whether MRI metrics of age-related damage were predictive of clinical progression. MRI results were corrected using the Monte Carlo simulation method, and regression analyses were further corrected for disease duration.
Results
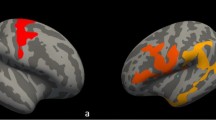
ALS patients exhibited significant CA mainly encompassing motor regions, but also involving the cuneus bilaterally and the right superior parietal cortex (p < 0.05). Older age at symptom onset was selectively associated with greater extra-motor (frontotemporal) CT, including pars opercularis bilaterally, left middle temporal, and parahippocampal cortices (p < 0.05), and CT of these regions was predictive of shorter survival (p = 0.004, p = 0.03).
Conclusion
More severe frontotemporal CT contributes to shorter survival in older ALS patients. These findings have the potential to unravel the neurobiological mechanisms linking older age at symptom onset to worse prognosis in ALS.



Similar content being viewed by others
Code availability
Not applicable.
Data Availability
Raw data are available upon appropriate request.
References
Longinetti E, Fang F (2019) Epidemiology of amyotrophic lateral sclerosis: an update of recent literature. Curr Opin Neurol 32(5):771–776. https://doi.org/10.1097/WCO.0000000000000730
Turner MR, Barnwell J, Al-Chalabi A, Eisen A (2012) Young-onset amyotrophic lateral sclerosis: historical and other observations. Brain 135(Pt 9):2883–2891. https://doi.org/10.1093/brain/aws144
Renton AE, Chiò A, Traynor BJ (2014) State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 17(1):17–23. https://doi.org/10.1038/nn.3584
Lomen-Hoerth C, Murphy J, Langmore S, Kramer JH, Olney RK, Miller B (2003) Are amyotrophic lateral sclerosis patients cognitively normal? Neurology 60(7):1094–1097. https://doi.org/10.1212/01.wnl.0000055861.95202.8d
Irwin D, Lippa CF, Swearer JM (2007) Cognition and amyotrophic lateral sclerosis (ALS). Am J Alzheimers Dis Other Dement 22(4):300–312. https://doi.org/10.1177/1533317507301613
Montuschi A, Iazzolino B, Calvo A, Moglia C, Lopiano L, Restagno G, Brunetti M, Ossola I, Lo Presti A, Cammarosano S, Canosa A, Chio A (2015) Cognitive correlates in amyotrophic lateral sclerosis: a population-based study in Italy. J Neurol Neurosurg Psychiatry 86(2):168–173. https://doi.org/10.1136/jnnp-2013-307223
Chio A, Logroscino G, Hardiman O, Swingler R, Mitchell D, Beghi E et al (2009) Prognostic factors in ALS: a critical review. Amyotroph Lateral Scler 10(5-6):310–323. https://doi.org/10.3109/17482960802566824
Calvo A, Moglia C, Lunetta C, Marinou K, Ticozzi N, Ferrante DG et al (2017) Factors predicting survival in ALS: a multicenter Italian study. J Neurol 264(1):54–63. https://doi.org/10.1007/s00415-016-8313-y
Grosskreutz J, Kaufmann J, Frädrich J, Dengler R, Heinze HJ, Peschel T (2006) Widespread sensorimotor and frontal cortical atrophy in amyotrophic lateral sclerosis. BMC Neurol 6:17. https://doi.org/10.1186/1471-2377-6-17
Agosta F, Ferraro PM, Riva N, Spinelli EG, Chio’ A, Canu E et al (2016) Structural brain correlates of cognitive and behavioral impairment in MND. Hum Brain Mapp 37(4):1614–1626. https://doi.org/10.1002/hbm.23124
Schuster C, Kasper E, Machts J, Bittner D, Kaufmann J, Benecke R, Teipel S, Vielhaber S, Prudlo J (2014) Longitudinal course of cortical thickness decline in amyotrophic lateral sclerosis. J Neurol 261(10):1871–1880. https://doi.org/10.1007/s00415-014-7426-4
Walhout R, Westeneng HJ, Verstraete E, Hendrikse J, Veldink JH, van den Heuvel MP, van den Berg LH (2015) Cortical thickness in ALS: towards a marker for upper motor neuron involvement. J Neurol Neurosurg Psychiatry 86(3):288–294. https://doi.org/10.1136/jnnp-2013-306839
Senda J, Atsuta N, Watanabe H, Bagarinao E, Imai K, Yokoi D, Riku Y, Masuda M, Nakamura R, Watanabe H, Ito M, Katsuno M, Naganawa S, Sobue G (2017) Structural MRI correlates of amyotrophic lateral sclerosis progression. J Neurol Neurosurg Psychiatry 88(11):901–907. https://doi.org/10.1136/jnnp-2016-314337
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1(5):293–299. https://doi.org/10.1080/146608200300079536
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 169(1-2):13–21. https://doi.org/10.1016/s0022-510x(99)00210-5
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149(2):351–356. https://doi.org/10.2214/ajr.149.2.351
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97(20):11050–11055. https://doi.org/10.1073/pnas.200033797
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9(2):179–194. https://doi.org/10.1006/nimg.1998.0395
Mezzapesa DM, D’Errico E, Tortelli R, Distaso E, Cortese R, Tursi M, Federico F, Zoccolella S, Logroscino G, Dicuonzo F, Simone IL (2013) Cortical thinning and clinical heterogeneity in amyotrophic lateral sclerosis. PLoS One 8(11):e80748. https://doi.org/10.1371/journal.pone.0080748
Bede P, Bokde A, Elamin M, Byrne S, McLaughlin RL, Jordan N et al (2013) Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): a neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. J Neurol Neurosurg Psychiatry 84(7):766–773. https://doi.org/10.1136/jnnp-2012-302674
Qin Y, Zhang S, Jiang R, Gao F, Tang X, Zhu W (2018) Region-specific atrophy of precentral gyrus in patients with amyotrophic lateral sclerosis. J Magn Reson Imaging 47(1):115–122. https://doi.org/10.1002/jmri.25765
Chow TW, Binns MA, Freedman M, Stuss DT, Ramirez J, Scott CJ et al (2008) Overlap in frontotemporal atrophy between normal aging and patients with frontotemporal dementias. Alzheimer Dis Assoc Disord 22(4):327–335. https://doi.org/10.1097/WAD.0b013e31818026c4
Omer T, Finegan E, Hutchinson S, Doherty M, Vajda A, McLaughlin RL et al (2017) Neuroimaging patterns along the ALS-FTD spectrum: a multiparametric imaging study. Amyotroph Lateral Scler Frontotemporal Degener 18(7-8):611–623. https://doi.org/10.1080/21678421.2017.1332077
Lillo P, Mioshi E, Burrell JR, Kiernan MC, Hodges JR, Hornberger M (2012) Grey and white matter changes across the amyotrophic lateral sclerosis-frontotemporal dementia continuum. PLoS One 7(8):e43993. https://doi.org/10.1371/journal.pone.0043993
Consonni M, Contarino VE, Catricala’ E, Dalla Bella E, Pensato V, Gellera C et al (2018) Cortical markers of cognitive syndromes in amyotrophic lateral sclerosis. Neuroimage Clin 19:675–682. https://doi.org/10.1016/j.nicl.2018.05.020
Shellikeri S, Myers M, Black SE, Abrahao A, Zinman L, Yusunova Y (2019) Speech network regional involvement in bulbar ALS: a multimodal structural MRI study. Amyotroph Lateral Scler Frontotemporal Degener 20(5-6):385–395. https://doi.org/10.1080/21678421.2019.1612920
Rhinn H, Abeliovich A (2017) Differential aging analysis in human cerebral cortex identifies variants in TMEM106B and GRN that regulate aging phenotypes. Cell Syst 4(4):404–15.e5. https://doi.org/10.1016/j.cels.2017.02.009
Pandya VA, Patani R (2020) Decoding the relationship between ageing and amyotrophic lateral sclerosis: a cellular perspective. Brain 143(4):1057–1072. https://doi.org/10.1093/brain/awz360
Acknowledgements
The authors are grateful to patients and healthy volunteers for participating in the study.
Funding
This work was supported by grants from Italian Ministry of Health (5x1000 2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethic approval
Retrospective analysis of the data was approved by the IRCCS Ospedale Policlinico San Martino Review Board.
Consent to participate
Written informed consent was obtained from each participant according to the institution’s procedures and the Declaration of Helsinki.
Consent for publication
Not applicable.
Conflict of interest
We declare that we have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Figure 1.
Three-dimensional reconstructed MR imaging maps show regions of significant cortical atrophy on the inflated surface in the left (L) and right (R) hemispheres in ALS patients (excluding the two genetic cases) compared to healthy controls (Montecarlo, p< 0.05). (JPG 273 kb)
Supplementary Figure 2.
Three-dimensional reconstructed MR imaging maps show regions of significant cortical thinning related to older age at symptoms onset on the inflated surface in the left (L) and right (R) hemispheres in ALS patients (excluding the two genetic cases) (Montecarlo, p< 0.05). (JPG 322 kb)
Rights and permissions
About this article
Cite this article
Ferraro, P.M., Cabona, C., Meo, G. et al. Age at symptom onset influences cortical thinning distribution and survival in amyotrophic lateral sclerosis. Neuroradiology 63, 1481–1487 (2021). https://doi.org/10.1007/s00234-021-02681-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02681-3