Abstract
Purpose
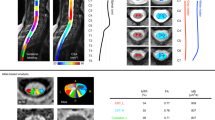
Reduced gray-white matter contrast along the central sulcus has been described on T1- and T2-weighted magnetic resonance imaging (MRI). The purpose of this study was to assess the gray-white matter contrast of the motor cortex on double inversion recovery (DIR), a sequence with superior gray-white matter differentiation.
Methods
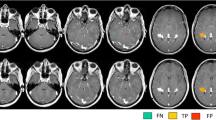
The gray-white matter signal on DIR was retrospectively compared to T1-weighted magnetization-prepared rapid gradient echo (T1-MPRAGE) using normal (n = 25) and abnormal (n = 25) functional MRI (fMRI) exams. Quantitative gray-white matter contrast ratios (CR) of the precentral and adjacent gyri were obtained on normal exams. Two neuroradiologists qualitatively rated reduced gray-white matter contrast of the hemispheres of both normal and abnormal exams. Hand motor functional mapping was used as a reference.
Results
In normal hemispheres (n = 50), the mean CR was significantly lower on DIR (0.44) vs T1-MPRAGE (0.63, p < 0.001). Reduced gray-white matter contrast was categorized as “definitely present” more frequently on DIR than T1-MPRAGE by reviewers in both normal (n = 50; reviewer 1 DIR 88% and MPRAGE 68%, p = 0.02; reviewer 2 DIR 86% and T1-MPRAGE 64%; p=0.01) and abnormal hemispheres (n = 50; reviewer 1 DIR 80% and T1-MPRAGE 38%, p < 0.001; reviewer 2 DIR 74% and T1-MPRAGE 46%, p = 0.005).
Conclusion
Reduced gray-white matter contrast of the motor cortex is more pronounced on DIR compared to T1-MPRAGE on quantitative and qualitative assessments of normal MRI exams. In abnormal cases, reviewers more definitively identified the motor cortex on DIR. In cases with distorted brain anatomy, DIR may be a useful adjunct sequence to localize the motor cortex.




Similar content being viewed by others
References
Mahdavi A, Azar R, Shoar MH, Hooshmand S, Mahdavi A, Kharrazi HH (2015) Functional MRI in clinical practice: assessment of language and motor for pre-surgical planning. Neuroradiol J 28:468–473
Mahvash M, Maslehaty H, Jansen O, Mehdorn HM, Petridis AK (2014) Functional magnetic resonance imaging of motor and language for preoperative planning of neurosurgical procedures adjacent to functional areas. Clin Neurol Neurosurg 123:72–77
Tieleman A, Deblaere K, Van Roost D et al (2009) Preoperative fMRI in tumour surgery. Eur Radiol 19:2523–2534
Biega TJ, Lonser RR, Butman JA (2006) Differential cortical thickness across the central sulcus: a method for identifying the central sulcus in the presence of mass effect and vasogenic edema. AJNR Am J Neuroradiol 27:1450–1453
Sunaert S (2006) Presurgical planning for tumor resectioning. J Magn Reson Imaging 23:887–905
Sahin N, Mohan S, Maralani PJ, Duddukuri S, O’Rourke DM, Melhem ER, Wolf RL (2016) Assignment confidence in localization of the hand motor cortex: comparison of structural imaging with functional MRI. AJR Am J Roentgenol 207:1263–1270
Meyer JR, Roychowdhury S, Russell EJ, Callahan C, Gitelman D, Mesulam MM (1996) Location of the central sulcus via cortical thickness of the precentral and postcentral gyri on MR. AJNR Am J Neuroradiol 17:1699–1706
Kido DK, LeMay M, Levinson AW, Benson WE (1980) Computed tomographic localization of the precentral gyrus. Radiology 135:373–377
Yousry TA, Schmid UD, Alkadhi H et al (1997) Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120(Pt 1):141–157
Ebeling U, Huber P, Reulen HJ (1986) Localization of the precentral gyrus in the computed tomogram and its clinical application. J Neurol 233:73–76
Kaneko OF, Fischbein NJ, Rosenberg J et al (2017) The “white gray sign” identifies the central sulcus on 3T high-resolution T1-weighted images. AJNR Am J Neuroradiol 38:276–280
Kamada K, Kakeda S, Ohnari N, Moriya J, Sato T, Korogi Y (2008) Signal intensity of motor and sensory cortices on T2-weighted and FLAIR images: intraindividual comparison of 1.5T and 3T MRI. Eur Radiol 18:2949–2955
Kakeda S, Korogi Y, Kamada K, Ohnari N, Moriya J, Sato T, Kitajima M, Hasnine H, Hirata N (2008) Signal intensity of the motor cortex on phase-weighted imaging at 3T. AJNR Am J Neuroradiol 29:1171–1175
Wattjes MP, Lutterbey GG, Gieseke J, Träber F, Klotz L, Schmidt S, Schild HH (2007) Double inversion recovery brain imaging at 3T: diagnostic value in the detection of multiple sclerosis lesions. AJNR Am J Neuroradiol 28:54–59
Wong-Kisiel LC, Britton JW, Witte RJ, Kelly-Williams KM, Kotsenas AL, Krecke KN, Watson RE Jr, Patton A, Hanson DP, Mandrekar J (2016) Double inversion recovery magnetic resonance imaging in identifying focal cortical dysplasia. Pediatr Neurol 61:87–93
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Amunts K, Zilles K (2015) Architectonic mapping of the human brain beyond Brodmann. Neuron 88:1086–1107
Fukunaga M, Li T-Q, van Gelderen P, de Zwart JA, Shmueli K, Yao B, Lee J, Maric D, Aronova MA, Zhang G, Leapman RD, Schenck JF, Merkle H, Duyn JH (2010) Layer-specific variation of iron content in cerebral cortex as a source of MRI contrast. Proc Natl Acad Sci U S A 107:3834–3839
Fatterpekar GM, Naidich TP, Delman BN, Aguinaldo JG, Gultekin SH, Sherwood CC, Hof PR, Drayer BP, Fayad ZA (2002) Cytoarchitecture of the human cerebral cortex: MR microscopy of excised specimens at 9.4 tesla. AJNR Am J Neuroradiol 23:1313–1321
Glasser MF, Goyal MS, Preuss TM, Raichle ME, van Essen DC (2014) Trends and properties of human cerebral cortex: correlations with cortical myelin content. Neuroimage 93(Pt 2):165–175
Steen RG, Gronemeyer SA, Taylor JS (1995) Age-related changes in proton T1 values of normal human brain. J Magn Reson Imaging 5:43–48
Ballesteros MC, Hansen PE, Soila K (1993) MR imaging of the developing human brain. Part 2. Postnatal development. Radiographics 13:611–622
Kim JA, Chung JI, Yoon PH, Kim DI, Chung TS, Kim EJ, Jeong EK (2001) Transient MR signal changes in patients with generalized tonicoclonic seizure or status epilepticus: periictal diffusion-weighted imaging. AJNR Am J Neuroradiol 22:1149–1160
Funding
This research was not funded.
Author information
Authors and Affiliations
Contributions
Conceptualization: Ranling Hu, Micheal J. Hoch, Jon T. Willie; methodology: Ranling Hu, Micheal J. Hoch, Jon T. Willie, Hena Joshi; formal analysis and investigation: Ranling Hu, Micheal J. Hoch, Hena Joshi; writing—original draft preparation: Hena Joshi, Maria Braileanu; writing—review and editing: Ranling Hu, Micheal J. Hoch, Jon T. Willie, Hena Joshi, Maria Braileanu, Ashwani Gore; supervision: Ranling Hu, Hena Joshi, Maria Braileanu; data availability. All data and materials support our claims and comply with field standards.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This research study was conducted retrospectively from data obtained for clinical purposes. This study was approved by the Emory University institutional review board (IRB) with a waiver for informed consent.
Consent to participate
This was a retrospective study approved by the Emory University Institutional Review Board (IRB); consent to participate was not obtained.
Consent for publication
This was a retrospective study approved by the Emory University Institutional Review Board (IRB); consent for publication was not obtained.
Code availability
No special code was created for this study; methods were performed with readily available software.
Additional information
Presentation
Previously presented as a poster at 12th Annual Meeting of the American Society of Functional Neuroradiology, October 15-17, 2018; Coronado, California, USA.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Joshi, H., Hoch, M.J., Braileanu, M. et al. Reduced gray-white matter contrast localizes the motor cortex on double inversion recovery (DIR) 3T MRI. Neuroradiology 63, 1071–1078 (2021). https://doi.org/10.1007/s00234-020-02631-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02631-5