Abstract
Purpose
This study aims to determine local diagnostic reference levels (LDRLs) of intra-arterial chemotherapy (IAC) procedures of pediatric patients with retinoblastoma (RB) to provide data for establishing diagnostic reference levels (DRLs) in pediatric interventional radiology (IR).
Methods
In a retrospective study design, LDRLs and achievable dose (AD) were assessed for children undergoing superselective IAC for RB treatment. All procedures were performed at the flat-panel angiography systems (I) ArtisQ biplane (Siemens Healthineers) and (II) Allura Xper (Philips Healthcare). Patients were differentiated according to age (A1: 1–3 months; A2: 4–12 months; A3: 13–72 months; A4: 73 months–10 years; A5: > 10 years), sex, conducted or not-conducted chemotherapy.
Results
248 neurointerventional procedures of 130 pediatric patients (median age 14.5 months, range 5–127 months) with RB (68 unilateral, 62 bilateral) could be included between January 2010 and March 2020. The following diagnostic reference values, AD, and mean values could be determined: (A2) DRL 3.9 Gy cm2, AD 2.9 Gy cm2, mean 3.5 Gy cm2; (A3) DRL 7.0 Gy cm2, AD 4.3 Gy cm2, mean 6.0 Gy cm2; (A4) DRL 14.5 Gy cm2, AD 10.7 Gy cm2, mean 10.8 Gy cm2; (A5) AD 8.8 Gy cm2, mean 8.8 Gy cm2. Kruskal-Wallis-test confirmed a significant dose difference between the examined age groups (A2–A5) (p < 0.001). There was no statistical difference considering sex (p = 0.076) and conducted or not-conducted chemotherapy (p = 0.627). A successful procedure was achieved in 207/248 cases.
Conclusion
We report on radiation exposure during superselective IAC of a pediatric cohort at the German Retinoblastoma Referral Centre. Although an IAC formally represents a therapeutic procedure, our results confirm that radiation exposure lies within the exposure of a diagnostic interventional procedure. DRLs for superselective IAC are substantially lower compared with DRLs of more complex endovascular interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of pediatric interventional radiology (IR) procedures has increased over the last decade. These procedures are less common in the pediatric population but may comprise high radiation doses. In general, in pediatric patients, the developing organs and tissues are more sensitive to the harmful effects of radiation and the longer life expectancy increases the risk for developing radiation-induced cancer compared with that in adults receiving the same dose [1]. Nevertheless, pediatric international diagnostic reference levels (DRLs) are lacking for IR, especially for cerebral angiography.
DRLs are a vital element for dose monitoring and are globally accepted in order to achieve dose optimization in the clinical routine. They represent the 75th percentile of a dose distribution of a specific radiological procedure and may indicate whether the radiation dose lies within the normal range of a dose distribution at radiological departments [2, 3]. The achievable dose (AD) represents the 50th percentile of a dose distribution and may serve as another parameter for dose optimization [3, 4]. Therefore, the International Commission on Radiological Protection (ICRP) and the European Guidelines on Diagnostic Reference Levels for Pediatric Imaging are proclaiming the necessity for DRLs for pediatric patients [1, 5]. It is expected in the pediatric radiology community that pediatric DRLs will increase dose awareness and in the long term optimize the modification of equipment, technique, and imaging parameters.
In the last decade, intra-arterial chemotherapy (IAC) as an IR procedure has been applied increasingly in the clinical management of retinoblastoma. IAC is used both as primary and secondary treatment of retinoblastoma and it is reported to provide tumor control even in advanced-stage disease that might have previously required enucleation [6, 7]. Various studies demonstrated the possible benefits of this treatment, especially less systematic side effects and a lower rate of bulbus loss and relapse [8,9,10]. Thus, superselective IAC is more and more used in children with retinoblastoma (RB) for an eye preserving approach. However, superselective IAC always involves exposure to X-rays. This issue weighs even more considering the fact that the RB gene mutation itself represents a genetic predisposition to malignancy [11, 12].
Until now, little published data exists to our knowledge discussing radiation exposure of superselective, intra-arterial melphalan therapy in children with RB [13, 14]. Hence, the purpose of this study was to evaluate radiation exposure for children undergoing traditional guidewire-directed, superselective chemotherapy for RB treatment as a function of age, sex, and interventional success and to establish local diagnostic reference levels (LDRLs) as a function of age.
Materials and methods
Patient cohort
This retrospective study was approved by the internal ethical committee of our institution (20-9187-BO). Due to the retrospective nature of this study, no informed consent was required. All patient data were anonymized. The internal database of the radiology department of the University Hospital Essen was searched with an in-house-developed software for all IACs on retinoblastoma that were performed in the period between January 2010 and March 2020.
The cohort included patients with heritable and non-heritable RB, sporadic and familial RB, and uni-, bi- and trilateral tumors with ICRB grade B-E. Trilateral RB is a syndrome consisting of unilateral or bilateral hereditary RB associated with an intracranial neuroblastic tumor [15].
For cranial examinations, age is recommended as the grouping parameter in the European Guidelines on Diagnostic Reference Levels for Paediatric Imaging [5]. Following this, each examination was classified to one of five age groups (A1: 1–3 months; A2: 4–12 months; A3: 13–72 months; A4: 73 months −10 years; A5: > 10 years) depending on the patient age at the time of the interventional procedure. The distribution into the individual age groups is illustrated in Table 1.
Procedure
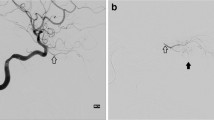
The standardized procedure is performed under general anaesthesia. A pediatric cardiologist provides transfemoral arterial access with a Doppler ultrasound–controlled needle in the Seldinger technique. The actual angiography examination by an interventional neuroradiologist comprises the following steps under fluoroscopic guidance (Fig. 1): (a) Placement of a 4-F guiding catheter into the cervical internal carotid artery and serial angiogram of the carotid artery in posterior/anterior and lateral projections is performed to visualize the cerebral and orbital angio-anatomy; (b) selective catheterization of the ostium of the ophthalmic artery with a microcatheter and injection of a contrast medium to confirm the correct position of the microcatheter and ascertain the lack of reflux into the internal carotid artery; hereby, choroid perfusion could be guaranteed, and meningeal collaterals excluded prior to intra-arterial melphalan therapy; (c) Injection of a weight-adapted chemotherapy under pressure control into the ophthalmic artery with a micro-perfusion pump over 30 min. Almost all our patients received a single-drug therapy with the cytostatic agent melphalan; only 2 patients received carboplatin alternatively. After 15 min, a fluoroscopic control was conducted to confirm an unchanged microcatheter system positioning; (d) at the end of the procedure prior to removal of the endovascular system, a final control angiogram is performed in posterior/anterior and lateral projections in order to rule out vasospasm and intracerebral complication. The standard procedure at our department is described in detail by Stenzel et al. [16]. The therapeutic regimen usually provides three sessions with an interval of 3–6 weeks and distance to previous polychemotherapy of at least 3 weeks [16].
Serial angiogram of the internal carotid artery (ICA) in posterior/anterior and lateral projections is performed to visualize the cerebral and orbital angio-anatomy, here exemplary p.a. projection (a). This angiogram is used for smart mask for superselective catheterization of the ostium of the ophthalmic artery with a microcatheter. Injection of a contrast medium to confirm the correct position of the microcatheter and ascertain the lack of reflux into the internal carotid artery; p.a. (b) and lateral (c) projection. Injection of a weight-adapted chemotherapy under pressure control into the ophthalmic artery with a micro-perfusion pump over 30 min. After 15 min, a fluoroscopic control was conducted to confirm an unchanged microcatheter system positioning; at the end of the procedure prior to removal of the endovascular system, a final control angiogram of the ICA is performed in posterior/anterior and lateral projections in order to rule out vasospasm and intracerebral complication, here exemplary p.a. projection (d)
Biplanar angiography system
Since January 2010, all consecutive procedures performed at the (II) Allura Xper FD20/10 system (Philips Healthcare, Eindhoven, The Netherlands) and since November 2018 supplementary at the (I) Artis Q biplane angiography system (Siemens Healthineers, Erlangen, Germany) were included. Both X-ray units are equipped with automatic control dose rate systems. According to clinical standards, the frame rate in both systems was one image per second for a scan time of approximately 8 s.
The focus-to-skin distance (FSD) varied from 60 to 70 cm. The Artis Q biplane angiography system (I) has two 20-inch detectors, each with a maximum FOV (field of view) of 48 cm. The Allura Xper system (II) has one detector 20-inch with a maximum FOV of 48 cm and one 10-inch detector with a maximum FOV of 25 cm. Gonadal protection is routinely applied. To test system performance and stability over time, periodic quality controls were performed in both systems during maintenance visits.
Endovascular system
Applied endovascular systems involved a Marathon-10 (Covidien/Medtronic, Inc., Mansfield, USA), HeadwayDuo (Microvention, Tustin, USA), or Echelon-10 microcatheter (Covidien/Medtronic, Inc., Mansfield, USA) as well as TransendEx-14 (Boston Scientific, Fremont, USA), Synchro-10 or Synchro-14 (both Stryker Neurovascular, Fremont, USA) microguidewires.
Statistical analysis
The mean, median, 75th percentile, and standard deviation of DAP and median FT were calculated. The procedures were analyzed according to the five age groups described above. A p value lower than 0.05 was considered statistically significant. The descriptive statistics and statistical analysis were performed with the Statistical Package for Social Sciences v. 26.0 (SPSS Inc., New York, USA).
Results
A total of 248 consecutive, neurointerventional procedures in 130 pediatric patients with retinoblastoma (68 unilateral; 62 bilateral; out of this 2 additional trilateral) were performed between January 2010 and March 2020. Of the 62 patients with bilateral RB, six patients received both left and right IAC in independent sessions. Patients received image-guided intra-arterial chemotherapy, either melphalan or carboplatin. The applied dose of melphalan (median 3 mg, 2.5–7.5 mg) as well as of carboplatin (30–40 mg) was adapted to weight and age. The median age of the patient cohort was 14.5 months (range, 5–127 months).
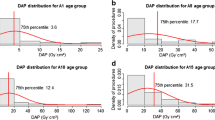
Radiation exposure was distributed as follows: (A2) DRL 3.9 Gy cm2, AD 2.9 Gy cm2, mean 3.5 Gy cm2; (A3) DRL 7.0 Gy cm2, AD 4.3 Gy cm2, mean 6.0 Gy cm2; (A4) DRL 14.5 Gy cm2, AD 10.7 Gy cm2, mean 10.8 Gy cm2; (A5) AD 8.8 Gy cm2, mean 8.8 Gy cm2 (Table 2). Kruskal-Wallis-test confirmed a significant dose difference between the examined age groups (A2–A5) (p < 0.001) (Fig. 2). No statistical difference for conducted or not-conducted chemotherapy (p = 0.627) (Fig. 3) and sex (p = 0.076) (Fig. 4a, b) was found. The average number of individual superselective IAC administrations was 1.984 (median 1.00). A successful neurointerventional procedure was achieved in 207/248 (83.5%) cases. In 41/248 sessions (16.5%) the therapeutic angiography had to be interrupted without injecting IAC for the following reasons: (1) significant collaterals to meningeal arteries (13 patients), (2) technical failure of ophthalmic artery catheterization (16 patients), or (3) retina blood supply from collaterals different to the ophthalmic artery (12 patients). Only in one case was there a periprocedural complication after intra-arterial melphalan application in terms of thromboembolism.
Discussion
Here, we present LDRLs of children undergoing superselective IAC for RB treatment at the German Retinoblastoma Referral Centre. Radiation exposure is of particular concern in children with heritable RB as they face a lifelong increased predisposition to second primary malignancies [17, 18].
The presented results show that the local diagnostic reference values and the achievable dose for superselective IAC therapy are substantially lower compared with DRLs of more complex endovascular interventions like thrombus aspiration (DRL 179.99 Gy cm2) or aneurysm coiling (DRL 249.99 Gy cm2) in adults, published by the Federal Office for Radiation Protection (Neuherberg, Germany) [19]. However, pediatric radiation dose is underestimated by the “one-size-fits-all” model [17]. Though several studies discussed a higher susceptibility of children to ionizing radiation [17, 18, 20,21,22,23], few authors addressed the issue of radiation exposure of pediatric interventional procedures at all, but in particular of superselective, intra-arterial melphalan therapies [13, 14] so far. The patient cohort described in these studies is limited to 11 [13] to 21 [14] sessions (including 8–16 patients). Vijayakrishnan et al. [13] described their preliminary data shortly after introducing this new technique [24]. Hence, it has to be postulated that they were able to reduce radiation exposure with increasing experience. Gobin et al. reported on only 14 superselective, intra-arterial melphalan therapies via the ophthalmic artery; 2 were performed via the middle meningeal artery and 5 in balloon technique [14]. Furthermore, radiation exposure was assessed by determining organ doses. This allows individual risk stratification. However, it is not practical in the clinical routine to compare radiation exposure of different devices and at different sites. Cooke et al. focused on the lens dose and the entrance skin dose in a small cohort (30 administrations) [25]. A recent study compared two techniques for ophthalmic chemotherapy administration [26]. The traditional guidewire-directed technique and the microcatheter-only technique as previously described by Gobin et al. [8], but with the addition of continuous verapamil infusion [26]. The authors focused on mean doses instead of on the DRLs.
The question about radiation exposure is of timeless concern in medical imaging [27], particularly in pediatric patients, who are generally more sensitive to radiation exposure [28]. Our analysis revealed that the DAP was not significantly higher during interventions in which an IAC could not be conducted due to an unsuccessful microcatheter and guide wire placement in the ophthalmic artery or meningeal anastomoses. However, all cases in which a chemotherapy could not be performed eventually result in an “unnecessary” radiation exposure. There was no difference of DAP concerning applied endovascular system.
In this context, DRLs are one of the main operational tools for detecting and optimizing image procedures in order to protect patients in radiological imaging. The DRLs were first recommended by the International Commission on Radiological Protection (ICRP) in 1991 [29] and some years later, in 1997, introduced in the European legislation by the Medical Exposure Directive 97/43/Euratom [30]. Since the Council Directive 2013/59/Euratom, all member states shall ensure that the established DRLs are regularly reviewed and used for optimization of protection [31]. Moreover, the Basic Safety Standards Directive expanded the application of DRLs to interventional radiology (IR) procedures [31]. Currently, only a few countries have set DRLs for pediatric examinations, and especially for pediatric interventional procedures, DRLs are rare. There are only few publications concerning patient dose for pediatric interventional cardiology [32,33,34,35]. For pediatric non-cardiologic interventional procedures, data is even scarcer. There is only one multicenter study from France publishing reference levels of three interventional neuroradiological procedures (cerebral digital subtraction angiography (DSA), embolization of brain arteriovenous malformation (bAVM), and percutaneous sclerotherapy of head and neck superficial vascular malformation (SVM)) [36]. Authors differentiated patient collective to different age groups: younger than 2 years (A1), aged 2–7 years (A5), 8–12 years (A10), and 13–18 years (A15). According to the study results, radiation exposures for a standard DSA were 4, 18, 12, and 32 Gy cm2, for bAVM 33, 70, 105, and 88 Gy cm2, and for SVM 350, 790, 490, and 248 m Gy cm2 in groups A1, A5, A10, and A15, respectively. Although an IAC formally represents a therapeutic procedure, our results confirm that radiation exposure lies within the exposure of a diagnostic interventional procedure.
Despite the fact that vein of Galen aneurysmal malformations represent the most common form of symptomatic cerebrovascular malformation in the early childhood, data on radiation exposure of therapeutic procedures in this special cohort are scarce. Curtis et al. examined the interventional procedure of a vein of Galen embolization of a 10-week-old by using a fluoroscopy fade technique [37]. The fluoroscopy fade technique is believed to comprise a lower contrast medium amount and a lower radiation exposure compared with the traditional road map–guided procedure. According to Curtis et al., the fluoroscopy fade technique in the vein of Galen embolization of the 10-week-old led to a skin entrance exposure of 480 mGy [37]. On the other hand, McParland reports a mean skin entrance dose of 100–110 mGy for standard p.a. and lateral projections as well as 340 mGy for standard embolization procedures [38]. Orbach et al. examined a total of 175 pediatric neurointerventions between September 2006 and July 2010 as well as 180 cases between July 2010 and June 2012 [39]. The examined neurointerventional procedures comprised several cerebrovascular pathologies such as brain AVM, pial fistulas, aneurysms, dural fistulas, and extracranial AVM or AVF, which were present in all age groups. The vein of Galen malformations were exclusively present in the < 1-year and 1- to 2-year age groups. According to Orbach et al., the maximal skin dose reached 372.9 mGy in the < 1-year-olds and 443.5 mGy in the1–2-year-olds [39].
For IR procedures, the patient dose depends on several factors, including the age and size of the patient, the complexity of the specific situation, and the experience of the medical staff. In general, therapeutic procedures have been reported to yield higher dose than diagnostic procedures [36]. Therefore, pediatric DRLs should be defined separately for specified diagnostic or therapeutic procedures [5].
Conclusion
RB represents one of the most frequent ocular malignancies in childhood. This is the first data acquisition of radiation exposure during superselective intra-arterial melphalan therapy of a pediatric cohort at a Retinoblastoma Referral Centre. Even if an IAC formally represents a therapeutic procedure, our results confirm that radiation exposure lies within the exposure of a diagnostic interventional procedure. Diagnostic reference values and the achievable dose for superselective IAC therapy are substantially lower compared with DRLs of more complex endovascular interventions. The examination evaluation of radiation exposure in a larger population and the comparison to international standards are the next necessary steps for the determination of national diagnostic reference levels (NDRLs) and European diagnostic reference levels (EDRLs) and to obtain a sufficient and reliable basis for implementing international pediatric DRLs which may ultimately contribute to radiation dose optimization in this rare but real entity.
Abbreviations
- AD:
-
Achievable dose
- DRLs:
-
Diagnostic reference levels
- DAP:
-
Dose area product
- EDRLs:
-
European diagnostic reference levels
- FOV:
-
Field of view
- IR:
-
Interventional radiology
- LDRLs:
-
Local diagnostic reference levels
- NDRLs:
-
National diagnostic reference levels
- RB:
-
Retinoblastoma
References
Khong P-L, Ringertz H, Donoghue V et al (2013) ICRP Publication 121: radiological protection in paediatric diagnostic and interventional radiology. Ann ICRP 42:1–63. https://doi.org/10.1016/j.icrp.2012.10.001
International Atomic Energy Agency (2014) Radiation protection and safety of radiation sources: international basic safety standards (GSR Part 3). International Atomic Energy Agency Vienna. /STI/PUB/1578
Guberina N, Forsting M, Suntharalingam S et al (2016) Radiation dose monitoring in the clinical routine. RöFo. https://doi.org/10.1055/s-0042-116684
National Council on Radiation Protection and Measurements (NCRP) (2012) Reference levels and achievable doses in medical and dental imaging : recommendations for the United States. Psychol Bull. https://doi.org/10.1037//0033-2909.I26.1.78
European Union (2018) Radiation protection (N°185), European Guidelines on Diagnostic Reference Levels for Paediatric Imaging. http://www.eurosafeimaging.org/wp/wp-content/uploads/2018/09/rp_185.pdf. Accessed 21 Jul 2020
Shields CL, Bianciotto CG, Jabbour P et al (2011) Intra-arterial chemotherapy for retinoblastoma: report no. 1, control of retinal tumors, subretinal seeds, and vitreous seeds. Arch Ophthalmol. https://doi.org/10.1001/archophthalmol.2011.150
Shields CL, Manjandavida FP, Lally SE et al (2014) Intra-arterial chemotherapy for retinoblastoma in 70 eyes: outcomes based on the international classification of retinoblastoma. Ophthalmology. https://doi.org/10.1016/j.ophtha.2014.01.026
Gobin YP, Dunkel IJ, Marr BP et al (2011) Intra-arterial chemotherapy for the management of retinoblastoma four-year experience. Arch Ophthalmol. https://doi.org/10.1001/archophthalmol.2011.5
Abramson DH (2010) Super selective ophthalmic artery delivery of chemotherapy for intraocular retinoblastoma: “chemosurgery” the first Stallard lecture. Br J Ophthalmol. https://doi.org/10.1136/bjo.2009.174268
Thampi S, Hetts SW, Cooke DL et al (2013) Superselective intra-arterial melphalan therapy for newly diagnosed and refractory retinoblastoma: results from a single institution. Clin Ophthalmol. https://doi.org/10.2147/OPTH.S43398
Wong FL, Boice JD, Abramson DH et al (1997) Cancer incidence after retinoblastoma: radiation dose and sarcoma risk. J Am Med Assoc. https://doi.org/10.1001/jama.278.15.1262
Kleinerman RA, Tucker MA, Tarone RE et al (2005) Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: an extended follow-up. J Clin Oncol. https://doi.org/10.1200/JCO.2005.05.054
Vijayakrishnan R, Shields CL, Ramasubramanian A et al (2010) Irradiation toxic effects during intra-arterial chemotherapy for retinoblastoma: should we be concerned? Arch Ophthalmol. https://doi.org/10.1001/archophthalmol.2010.258
Gobin YP, Rosenstein LM, Marr BP et al (2012) Radiation exposure during intra-arterial chemotherapy for retinoblastoma. Arch Ophthalmol. https://doi.org/10.1001/archopthalmol.2011.2717
Antoneli CBG, Ribeiro KDCB, Sakamoto LH et al (2007) Trilateral retinoblastoma. Pediatr Blood Cancer. https://doi.org/10.1002/pbc.20793
Stenzel E, Göricke S, Temming P et al (2019) Feasibility of intra-arterial chemotherapy for retinoblastoma: experiences in a large single center cohort study. Neuroradiology. https://doi.org/10.1007/s00234-019-02153-9
Strauss KJ, Goske MJ, Frush DP et al (2009) Image Gently vendor summit: working together for better estimates of pediatric radiation dose from CT. Am J Roentgenol. https://doi.org/10.2214/AJR.08.2172
Brenner DJ, Elliston CD, Hall EJ, Berdon WE (2001) Estimated risks of radiation-induced fatal cancer from pediatric CT. Am J Roentgenol. https://doi.org/10.2214/ajr.176.2.1760289
Schegerer A, Loose R, Heuser L, Brix G (2019) Diagnostische Referenzwerte für diagnostische und interventionelle Röntgenanwendungen in Deutschland: Aktualisierung und Handhabung. Fortschr Röntgenstr. https://doi.org/10.1055/a-0824-7603
Hintenlang KM, Williams JL, Hintenlang DE (2002) A survey of radiation dose associated with pediatric plain-film chest X-ray examinations. Pediatr Radiol. https://doi.org/10.1007/s00247-002-0734-3
Mettler J, Wiest PW, Locken JA, Kelsey CA (2000) CT scanning: patterns of use and dose. J Radiol Protect. https://doi.org/10.1088/0952-4746/20/4/301
Andreassi MG, Picano E (2014) Reduction of radiation to children our responsibility to change. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.114.010699
Brenner DJ, Hall EJ (2007) Computed tomography - an increasing source of radiation exposure. N Engl J Med. https://doi.org/10.1056/NEJMra072149
Shields CL, Vijayakrishnan R, Ramasubramanian A et al (2012) Radiation exposure during intra-arterial chemotherapy for retinoblastoma - reply. Arch Ophthalmol. https://doi.org/10.1001/archopthalmol.2012.173
Cooke DL, Stout CE, Kim WT et al (2014) Radiation dose reduction in intra-arterial chemotherapy infusion for intraocular retinoblastoma. J Neuro Interv Surg. https://doi.org/10.1136/neurintsurg-2013-010905
Area C, Yen CJ, Chevez-Barrios P et al (2019) Technical and anatomical factors affecting intra-arterial chemotherapy fluoroscopy time and radiation dose for intraocular retinoblastoma. J Neuro Interv Surg. https://doi.org/10.1136/neurintsurg-2019-014910
National Research Council (2006) Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2. https://doi.org/10.17226/11340
Pearce MS, Salotti JA, Little MP et al (2012) Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. https://doi.org/10.1016/S0140-6736(12)60815-0
Mountford PJ, Temperton DH (1992) Recommendations of the International Commission on Radiological Protection (ICRP) 1990. Eur J Nucl Med. https://doi.org/10.1007/BF00184120
Teunen D (1998) The European Directive on health protection of individuals against the dangers of ionising radiation in relation to medical exposures (97/43/EURATOM). J Radiol Protect. https://doi.org/10.1088/0952-4746/18/2/009
Council of the European Union (2013) Council Directive 2013/59/Euratom. Off J Eur Union. https://doi.org/10.3000/19770677.L_2013.124.eng
Buytaert D, Vandekerckhove K, Panzer J et al (2019) Local DRLs and automated risk estimation in paediatric interventional cardiology. PLoS One. https://doi.org/10.1371/journal.pone.0220359
McFadden S, Hughes C, D’Helft CI et al (2013) The establishment of local diagnostic reference levels for paediatric interventional cardiology. Radiography. https://doi.org/10.1016/j.radi.2013.04.006
Corredoira E, Vañó E, Ubeda C, Gutiérrez-Larraya F (2015) Patient doses in paediatric interventional cardiology: impact of 3D rotational angiography. J Radiol Protect. https://doi.org/10.1088/0952-4746/35/1/179
Barnaoui S, Rehel JL, Baysson H et al (2014) Local reference levels and organ doses from pediatric cardiac interventional procedures. Pediatr Cardiol. https://doi.org/10.1007/s00246-014-0895-5
Habib Geryes B, Bak A, Lachaux J et al (2017) Patient radiation doses and reference levels in pediatric interventional radiology. Eur Radiol. https://doi.org/10.1007/s00330-017-4769-0
Given CA, Thacker IC, Baker MD, Morris PP (2003) Fluoroscopy fade for embolization of vein of Galen malformation. Am J Neuroradiol
Mcparland BJ (1998) Entrance skin dose estimates derived from dose-area product measurements in interventional radiological procedures. Br J Radiol. https://doi.org/10.1259/bjr.71.852.10319003
Orbach DB, Stamoulis C, Strauss KJ et al (2014) Neurointerventions in children: radiation exposure and its import. Am J Neuroradiol. https://doi.org/10.3174/ajnr.A3758
Acknowledgements
Open Access funding provided by Projekt DEAL.
Funding
No funding was received for this study. D. Bos was supported as a Clinician Scientist within the University Medicine Essen Academy (UMEA) program, funded by the German Research Foundation (DFG; grant FU356/12-1) and the Faculty of Medicine, University of Duisburg-Essen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of retrospective study, formal consent is not required and all patient data was anonymized.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Opitz, M., Bos, D., Deuschl, C. et al. Estimation of radiation exposure of children undergoing superselective intra-arterial chemotherapy for retinoblastoma treatment: assessment of local diagnostic reference levels as a function of age, sex, and interventional success. Neuroradiology 63, 391–398 (2021). https://doi.org/10.1007/s00234-020-02540-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02540-7