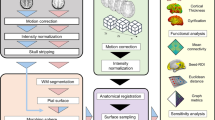
Abstract
Purpose
Spatial smoothing is an essential pre-processing step in the process of analysing functional magnetic resonance imaging (fMRI) data, both during an experimental task or during resting-state fMRI (rsfMRI). The main benefit of this spatial smoothing step is to artificially increase the signal-to-noise ratio of the fMRI signal. Previous fMRI studies have investigated the impact of spatial smoothing on task fMRI data, while rsfMRI studies usually apply the same analytical process used for the task data. However, this study investigates changes in different rsfMRI analyses, such as ROI-to-ROI, seed-to-voxels and ICA analyses.
Methods
Nineteen healthy volunteers were scanned using rsfMRI with three applied smoothing kernels: 0 mm, 4 mm and 8 mm. Appropriate statistical comparisons were made.
Results
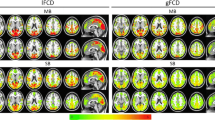
The findings showed that spatial smoothing has a greater effect on rsfMRI data when analysed using seed-to-voxel-based analysis. The effect was less pronounced when analysing data using ROI-ROI or ICA analyses. The results demonstrated that even when analysing the data without the application of spatial smoothing, the results were significant compared with data analysed using a typical smoothing kernel. However, data analysed with lower-smoothing kernels produced greater negative correlations, particularly with the ICA analysis.
Conclusion
The results suggest that a medium smoothing kernel (around 4 mm) may be preferable, as it is comparable with the 8 mm kernel in all of the analyses performed. It is also recommended that the researchers consider analysing the data using two different smoothing kernels, as this will help to confirm the significance of the results and avoid overestimating the findings.





Similar content being viewed by others
References
Friston K (2002) Functional integration and inference in the brain. Prog Neurobiol 68(2):113–143
Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34(4):537–541
Reimann M, Schilke O, Weber B, Neuhaus C, Zaichkowsky J (2011) Functional magnetic resonance imaging in consumer research: a review and application. Psychol Mark 28(6):608–637
Lee MH, Smyser CD, Shimony JS (2013) Resting-state fMRI: a review of methods and clinical applications. Am J Neuroradiol 34(10):1866–1872
Logothetis NK (2008) What we can do and what we cannot do with fMRI. Nature 453(7197):869–878. https://doi.org/10.1038/nature06976
Smith SM, Vidaurre D, Beckmann CF, Glasser MF, Jenkinson M, Miller KL, Nichols TE, Robinson EC, Salimi-Khorshidi G, Woolrich MW (2013) Functional connectomics from resting-state fMRI. Trends Cogn Sci 17(12):666–682
Smith SM (2004) Overview of fMRI analysis. Br J Radiol 77(suppl_2):S167–S175. https://doi.org/10.1259/bjr/33553595
Jezzard P, Toosy A (2005) Functional MRI. In: MR Imaging in White Matter Diseases of the Brain and Spinal Cord. Springer, pp 93–110
Smith SM, Matthews PM, Jezzard P (2001) Functional MRI: an introduction to methods. Oxford University Press, Oxford
Strother SC (2006) Evaluating fMRI preprocessing pipelines. IEEE Eng Med Biol Mag 25(2):27–41
Henson RN (2006) Efficient experimental design for fMRI. In: Friston K, Ashburner J, Kiebel S, Nichols T, Penny W, (eds) Statistical parametric mapping: the analysis of functional brain images. London, Elsevier, pp 193–210
Wang J, Wang Z, Aguirre GK, Detre JA (2005) To smooth or not to smooth? ROC analysis of perfusion fMRI data. Magn Reson Imaging 23(1):75–81
Margulies DS, Böttger J, Long X, Lv Y, Kelly C, Schäfer A, Goldhahn D, Abbushi A, Milham MP, Lohmann G (2010) Resting developments: a review of fMRI post-processing methodologies for spontaneous brain activity. MAGMA 23(5-6):289–307
Hagler DJ Jr, Saygin AP, Sereno MI (2006) Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. NeuroImage 33(4):1093–1103
Mikl M, Mareček R, Hluštík P, Pavlicová M, Drastich A, Chlebus P, Brázdil M, Krupa P (2008) Effects of spatial smoothing on fMRI group inferences. Magn Reson Imaging 26(4):490–503
Hopfinger JB, Büchel C, Holmes AP, Friston KJ (2000) A study of analysis parameters that influence the sensitivity of event-related fMRI analyses. NeuroImage 11(4):326–333
Bennett CM, Miller MB (2010) How reliable are the results from functional magnetic resonance imaging? Ann N Y Acad Sci 1191(1):133–155
Alakörkkö T, Saarimäki H, Glerean E, Saramäki J, Korhonen O (2017) Effects of spatial smoothing on functional brain networks. Eur J Neurosci 46(9):2471–2480
Smith SM (2001) 12 Preparing fMRI data for statistical analysis. Functional MRI: An introduction to methods:229
Wu CW, Chen C-L, Liu P-Y, Chao Y-P, Biswal BB, Lin C-P (2011) Empirical evaluations of slice-timing, smoothing, and normalization effects in seed-based, resting-state functional magnetic resonance imaging analyses. Brain Connect 1(5):401–410
Molloy EK, Meyerand ME, Birn RM (2014) The influence of spatial resolution and smoothing on the detectability of resting-state and task fMRI. NeuroImage 86:221–230
Worsley KJ (2003) Developments in Random Field Theory. (2):1-9
Alahmadi AA, Samson RS, Gasston D, Pardini M, Friston KJ, D'Angelo E, Toosy AT, Wheeler-Kingshott CA (2016) Complex motor task associated with non-linear BOLD responses in cerebro-cortical areas and cerebellum. Brain Struct Funct 221(5):2443–2458. https://doi.org/10.1007/s00429-015-1048-1
Boudrias MH, Goncalves CS, Penny WD, Park CH, Rossiter HE, Talelli P, Ward NS (2012) Age-related changes in causal interactions between cortical motor regions during hand grip. NeuroImage 59(4):3398–3405. https://doi.org/10.1016/j.neuroimage.2011.11.025
Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, Amunts K (2008) The human inferior parietal lobule in stereotaxic space. Brain Struct Funct 212(6):481–495. https://doi.org/10.1007/s00429-008-0195-z
Keisker B, Hepp-Reymond M-C, Blickenstorfer A, Meyer M, Kollias SS (2009) Differential force scaling of fine-graded power grip force in the sensorimotor network. Hum Brain Mapp 30(8):2453–2465. https://doi.org/10.1002/hbm.20676
Pajula J, Tohka J (2014) Effects of spatial smoothing on inter-subject correlation based analysis of FMRI. Magn Reson Imaging 32(9):1114–1124
Scheinost D, Papademetris X, Constable RT (2014) The impact of image smoothness on intrinsic functional connectivity and head motion confounds. NeuroImage 95:13–21
Tabelow K, Piëch V, Polzehl J, Voss HU (2009) High-resolution fMRI: overcoming the signal-to-noise problem. J Neurosci Methods 178(2):357–365. https://doi.org/10.1016/j.jneumeth.2008.12.011
Triantafyllou C, Hoge RD, Wald LL (2006) Effect of spatial smoothing on physiological noise in high-resolution fMRI. NeuroImage 32(2):551–557
Pizzagalli F, Auzias G, Delon-Martin C, Dojat M (2011) Combination of nonlinear registration methods with high resolution fMRI for a fine exploration of human primary motor hand area. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference 2011:6989–6992. https://doi.org/10.1109/IEMBS.2011.6091767
Lindquist MA, Loh JM, Yue YR (2010) Adaptive spatial smoothing of fMRI images. Statistics and its Interface 3(1):3–13
Liu Y, Liang P, Duan Y, Jia X, Yu C, Zhang M, Wang F, Zhang M, Dong H, Ye J, Butzkueven H, Li K (2011) Brain plasticity in relapsing-remitting multiple sclerosis: evidence from resting-state fMRI. J Neurol Sci 304(1-2):127–131. https://doi.org/10.1016/j.jns.2011.01.023
Parkes LM, Bastiaansen MC, Norris DG (2006) Combining EEG and fMRI to investigate the post-movement beta rebound. NeuroImage 29(3):685–696
Spraker MB, Yu H, Corcos DM, Vaillancourt DE (2007) Role of individual basal ganglia nuclei in force amplitude generation. J Neurophysiol 98(2):821–834. https://doi.org/10.1152/jn.00239.2007
Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S (2010) Toward discovery science of human brain function. Proc Natl Acad Sci 107(10):4734–4739
Khalili-Mahani N, Zoethout RM, Beckmann CF, Baerends E, de Kam ML, Soeter RP, Dahan A, van Buchem MA, van Gerven JM, Rombouts SA (2012) Effects of morphine and alcohol on functional brain connectivity during “resting state”: a placebo-controlled crossover study in healthy young men. Hum Brain Mapp 33(5):1003–1018
Klaassens BL, van Gorsel HC, Khalili-Mahani N, van der Grond J, Wyman BT, Whitcher B, Rombouts SA, van Gerven JM (2015) Single-dose serotonergic stimulation shows widespread effects on functional brain connectivity. NeuroImage 122:440–450
Whitfield-Gabrieli S, Nieto-Castanon A (2012) Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2(3):125–141. https://doi.org/10.1089/brain.2012.0073
Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25(4):1325–1335. https://doi.org/10.1016/j.neuroimage.2004.12.034
Chen Z, Calhoun V (2018) Effect of spatial smoothing on task fMRI ICA and functional connectivity. Front Neurosci 12(15). https://doi.org/10.3389/fnins.2018.00015
Zhang S, Li C-SR (2014) Functional clustering of the human inferior parietal lobule by whole-brain connectivity mapping of resting-state functional magnetic resonance imaging signals. Brain Connect 4(1):53–69
Skvortsova A, Veldhuijzen DS, de Rover M, Pacheco-Lopez G, Bakermans-Kranenburg M, van IJzendoorn M, Chavannes NH, van Middendorp H, Evers AW (2020) Effects of oxytocin administration and conditioned oxytocin on brain activity: an fMRI study. PLoS One 15(3):e0229692
Baldoli C, Scola E, Della Rosa PA, Pontesilli S, Longaretti R, Poloniato A, Scotti R, Blasi V, Cirillo S, Iadanza A (2015) Maturation of preterm newborn brains: a fMRI–DTI study of auditory processing of linguistic stimuli and white matter development. Brain Struct Funct 220(6):3733–3751
van der Miesen M (2015) The role of self-esteem in social feedback: an fMRI study. Student Undergraduate Research E-journal! 1
Kann S, Zhang S, Manza P, Leung H-C, Li C-SR (2016) Hemispheric lateralization of resting-state functional connectivity of the anterior insula: association with age, gender, and a novelty-seeking trait. Brain Connect 6(9):724–734
Gong J, Liu X, Liu T, Zhou J, Sun G, Tian J (2017) Dual temporal and spatial sparse representation for inferring group-wise brain networks from resting-state fMRI dataset. IEEE Trans Biomed Eng 65(5):1035–1048
Zarei M (2018) Precentral gyrus abnormal connectivity in male and female patients with schizophrenia
Schleifer C, Lin A, Kushan L, Ji JL, Yang G, Bearden CE, Anticevic A (2019) Dissociable disruptions in thalamic and hippocampal resting-state functional connectivity in youth with 22q11. 2 deletions. J Neurosci 39(7):1301–1319
Qing Z, Zhang X, Ye M, Wu S, Wang X, Nedelska Z, Hort J, Zhu B, Zhang B (2019) The impact of spatial normalization strategies on the temporal features of the resting-state functional MRI: spatial normalization before rs-fMRI features calculation may reduce the reliability. Front Neurosci 13:1249
Feis RA, Smith SM, Filippini N, Douaud G, Dopper EG, Heise V, Trachtenberg AJ, van Swieten JC, van Buchem MA, Rombouts SA (2015) ICA-based artifact removal diminishes scan site differences in multi-center resting-state fMRI. Front Neurosci 9:395
Dansereau C, Benhajali Y, Risterucci C, Pich EM, Orban P, Arnold D, Bellec P (2017) Statistical power and prediction accuracy in multisite resting-state fMRI connectivity. NeuroImage 149:220–232
Funding
This project was funded by the deanship of Scientific Research (DSR) at King Abdulaizz University (KAU), Jeddah. The author, therefore, acknowledges with thanks DSR for technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Ethical approval
The data samples were taken from the Leiden_2200 data sample (open access) (http://fcon_1000.projects.nitrc.org). All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. The included participant data were taken from the Leiden_2200 dataset which is part of the 1000 functional connectomes project.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alahmadi, A.A.S. Effects of different smoothing on global and regional resting functional connectivity. Neuroradiology 63, 99–109 (2021). https://doi.org/10.1007/s00234-020-02523-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02523-8