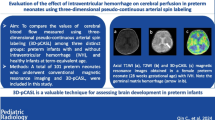
Abstract
Purpose
The long-term impact of low-grade germinal matrix-intraventricular hemorrhage (GMH-IVH) on brain perfusion has not been fully investigated. We aimed to compare cortical and deep gray matter (GM) cerebral blood flow (CBF) obtained with pseudo-continuous arterial spin labeling (pCASL), among preterm neonates with and without low-grade GMH-IVH and full-term controls.
Methods
3T-pCASL examinations of 9 healthy full-term neonates (mean gestational age 38.5 weeks, range 38–39) and 28 preterm neonates studied at term-equivalent age were analyzed. Eighteen preterm neonates presented normal brain MRI (mean gestational age 30.50 weeks, range 29–31) and 10 low-grade GMH-IVH according to Volpe’s grading system (mean gestational age 32 weeks, range 28–34). A ROI-based mean CBF quantification was performed in 5 cortical (frontal, parietal, temporal, insula, occipital), and 4 subcortical GM regions (caudate, putamen, pallidum, thalamus) for each cerebral hemisphere. CBF differences were explored using a nonparametric analysis of covariance.
Results
Low-grade GMH-IVH hemispheres showed consistently lower CBF in all GM regions when compared with healthy preterm neonates, after controlling the confounding effect of gestational age, postmenstrual age, and birth weight P < .001, η2 = .394. No significant differences were observed between neonates with low-grade GMH and full-term controls. Healthy preterm neonates showed significantly higher CBF than full-term controls in parietal (P = .032), temporal (P = .016), and occipital cortex (P = .024), and at level of thalamus (P = .023) and caudate nucleus (P = .014).
Conclusion
Low-grade GMH-IVH is associated with lower CBF in posterior cortical and subcortical gray matter regions in preterm neonates, suggesting regional vulnerability of these developing brain structures.




Similar content being viewed by others
Data Availability
Data used for the present analysis are available. For further information, please write to domenicotortora@gaslini.org.
Abbreviations
- ASL:
-
Arterial spin labeling
- CBF:
-
Cerebral blood flow
- GMH-IVH:
-
Germinal matrix and intraventricular hemorrhage
- HP:
-
Healthy preterm
- HT:
-
Healthy term
- pCASL:
-
Pseudo-continuous arterial spin labeling
- TEA:
-
Term-equivalent age
References
Sannia A, Natalizia AR, Parodi A, Malova M, Fumagalli M, Rossi A, Ramenghi LA (2015) Different gestational ages and changing vulnerability of the premature brain. J Matern Fetal Neonatal Med 28(Suppl 1):2268–2272
Ancel P-Y, Goffinet F, EPIPAGE-2 Writing Group, et al (2015) Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr 169:230–238
Brouwer AJ, Groenendaal F, Benders MJNL, de Vries LS (2014) Early and late complications of germinal matrix-intraventricular haemorrhage in the preterm infant: what is new? Neonatology 106:296–303
Klebermass-Schrehof K, Czaba C, Olischar M, Fuiko R, Waldhoer T, Rona Z, Pollak A, Weninger M (2012) Impact of low-grade intraventricular hemorrhage on long-term neurodevelopmental outcome in preterm infants. Childs Nerv Syst 28:2085–2092
Patra K, Wilson-Costello D, Taylor HG, Mercuri-Minich N, Hack M (2006) Grades I-II intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment. J Pediatr 149:169–173
Payne AH (2013) Neurodevelopmental outcomes of extremely low-gestational-age neonates with low-grade periventricular-intraventricular hemorrhage. JAMA Pediatr 167:451–459
Bolisetty S, Dhawan A, Abdel-Latif M, Bajuk B, Stack J, Lui K, on behalf of the New South Wales and Australian Capital Territory Neonatal Intensive Care Units’ Data Collection (2014) Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics 133:55–62
Beaino G, Khoshnood B, Kaminski M, Marret S, Pierrat V, Vieux R, Thiriez G, Matis J, Picaud JC, Rozé JC, Alberge C, Larroque B, Bréart G, Ancel PY, for the EPIPAGE Study Group (2011) Predictors of the risk of cognitive deficiency in very preterm infants: the EPIPAGE prospective cohort. Acta Paediatr 100:370–378
Beaino G, Khoshnood B, Kaminski M et al (2010) Predictors of cerebral palsy in very preterm infants: the EPIPAGE prospective population-based cohort study. Developmental Medicine & Child Neurology 52:e119–e125
Del Bigio MR (2011) Cell proliferation in human ganglionic eminence and suppression after prematurity-associated haemorrhage. Brain 134:1344–1361
Tortora D, Martinetti C, Severino M, Uccella S, Malova M, Parodi A, Brera F, Morana G, Ramenghi LA, Rossi A (2018) The effects of mild germinal matrix-intraventricular haemorrhage on the developmental white matter microstructure of preterm neonates: a DTI study. Eur Radiol 28:1157–1166
Lin P-Y, Hagan K, Fenoglio A, Grant PE, Franceschini MA (2016) Reduced cerebral blood flow and oxygen metabolism in extremely preterm neonates with low-grade germinal matrix- intraventricular hemorrhage. Sci Rep 6:25903
Verhagen EA, Ter Horst HJ, Keating P et al (2010) Cerebral oxygenation in preterm infants with germinal matrix-intraventricular hemorrhages. Stroke 41:2901–2907
Marin T, Moore J (2011) Understanding near-infrared spectroscopy. Advances in Neonatal Care 11:382–388
Miranda MJ, Olofsson K, Sidaros K (2006) Noninvasive measurements of regional cerebral perfusion in preterm and term neonates by magnetic resonance arterial spin labeling. Pediatr Res 60:359–363
Tortora D, Mattei PA, Navarra R, Panara V, Salomone R, Rossi A, Detre JA, Caulo M (2017) Prematurity and brain perfusion: arterial spin labeling MRI. NeuroImage: Clinical 15:401–407
Mahdi ES, Bouyssi-Kobar M, Jacobs MB, Murnick J, Chang T, Limperopoulos C (2018) Cerebral perfusion is perturbed by preterm birth and brain injury. AJNR Am J Neuroradiol 39:1330–1335
Chappell MA, Groves AR, Whitcher B, Woolrich MW (2009) Variational Bayesian inference for a nonlinear forward model. IEEE Trans Signal Process 57:223–236
Varela M, Hajnal JV, Petersen ET, Golay X, Merchant N, Larkman DJ (2011) A method for rapid in vivo measurement of blood T 1. NMR Biomed 24:80–88
De Vis JB, Hendrikse J, Groenendaal F et al (2014) Impact of neonate haematocrit variability on the longitudinal relaxation time of blood: implications for arterial spin labelling MRI. Neuroimage Clin 4:517–525
Inder TE, Perlman JM, Volpe JJ Preterm Intraventricular Hemorrhage/Posthemorrhagic Hydrocephalus In: Volpe JJ, Inder TE, Darras BT et al (2017) Volpe’s neurology of the newborn E-book. Elsevier Health Sciences pp 637–698.e21
Parodi A, Morana G, Severino MS, Malova M, Natalizia AR, Sannia A, Rossi A, Ramenghi LA (2015) Low-grade intraventricular hemorrhage: is ultrasound good enough? J Matern Fetal Neonatal Med 28:2261–2264
Childs AM, Ramenghi LA, Cornette L, Tanner SF, Arthur RJ, Martinez D, Levene MI (2001) Cerebral maturation in premature infants: quantitative assessment using MR imaging. AJNR Am J Neuroradiol 22:1577–1582
Tustison NJ, Avants BB, Cook PA, Yuanjie Zheng, Egan A, Yushkevich PA, Gee JC (2010) N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 29:1310–1320
Shi F, Yap P-T, Wu G, Jia H, Gilmore JH, Lin W, Shen D (2011) Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One 6:e18746
Mutsaerts HJ, Petr J, Václavů L et al (2017) The spatial coefficient of variation in arterial spin labeling cerebral blood flow images. J Cereb Blood Flow Metab 37:3184–3192
Tortora D, Scavetta C, Rebella G, Bertamino M, Scala M, Giacomini T, Morana G, Pavanello M, Rossi A, Severino M (2020) Spatial coefficient of variation applied to arterial spin labeling MRI may contribute to predict surgical revascularization outcomes in pediatric moyamoya vasculopathy. Neuroradiology 62:1003–1015
Verhagen EA, ter Horst HJ, Keating P, Martijn A, van Braeckel KNJA, Bos AF (2010) Cerebral oxygenation in preterm infants with germinal matrix–intraventricular hemorrhages. Stroke 41:2901–2907
Verhagen EA, Van Braeckel KNJA, van der Veere CN et al (2015) Cerebral oxygenation is associated with neurodevelopmental outcome of preterm children at age 2 to 3 years. Dev Med Child Neurol 57:449–455
Alderliesten T, Lemmers PMA, Smarius JJM et al (2013) Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J Pediatr 162:698–704.e2
Saha S, Pagnozzi A, Bourgeat P et al (2020) Predicting motor outcome in preterm infants from very early brain diffusion MRI using a deep learning convolutional neural network (CNN) model. Neuroimage 215:116807
Garcia KE, Robinson EC, Alexopoulos D, Dierker DL, Glasser MF, Coalson TS, Ortinau CM, Rueckert D, Taber LA, van Essen DC, Rogers CE, Smyser CD, Bayly PV (2018) Dynamic patterns of cortical expansion during folding of the preterm human brain. Proc Natl Acad Sci U S A 115:3156–3161
Hill J, Inder T, Neil J, Dierker D, Harwell J, van Essen D (2010) Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci U S A 107:13135–13140
Dubois J, Benders M, Cachia A, Lazeyras F, Ha-Vinh Leuchter R, Sizonenko SV, Borradori-Tolsa C, Mangin JF, Huppi PS (2008) Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex 18:1444–1454
Tanner SF, Ramenghi LA, Ridgway JP, Berry E, Saysell MA, Martinez D, Arthur RJ, Smith MA, Levene MI (2000) Quantitative comparison of intrabrain diffusion in adults and preterm and term neonates and infants. Am J Roentgenol 174:1643–1649
Argyropoulou MI, Astrakas LG, Xydis VG, Drougia A, Mouka V, Goel I, Giapros V, Andronikou S (2020) Is low-grade intraventricular hemorrhage in very preterm infants an innocent condition? Structural and functional evaluation of the brain reveals regional neurodevelopmental abnormalities. Am J Neuroradiol 41:542–547
Ceschin R, Wisnowski JL, Paquette LB, Nelson MD, Blüml S, Panigrahy A (2015) Developmental synergy between thalamic structure and interhemispheric connectivity in the visual system of preterm infants. Neuroimage Clin 8:462–472
Mukai T, Mori Y, Shimazu T, Takahashi A, Tsunoda H, Yamaguchi S, Kiryu S, Tojo A, Nagamura-Inoue T (2017) Intravenous injection of umbilical cord-derived mesenchymal stromal cells attenuates reactive gliosis and hypomyelination in a neonatal intraventricular hemorrhage model. Neuroscience 355:175–187
Xue M, Balasubramaniam J, Buist RJ, Peeling J, del Bigio MR (2003) Periventricular/intraventricular hemorrhage in neonatal mouse cerebrum. J Neuropathol Exp Neurol 62:1154–1165
Vinukonda G, Csiszar A, Hu F, Dummula K, Pandey NK, Zia MT, Ferreri NR, Ungvari Z, LaGamma EF, Ballabh P (2010) Neuroprotection in a rabbit model of intraventricular haemorrhage by cyclooxygenase-2, prostanoid receptor-1 or tumour necrosis factor-alpha inhibition. Brain 133:2264–2280
Truttmann AC, Ginet V, Puyal J (2020) Current evidence on cell death in preterm brain injury in human and preclinical models. Front Cell Dev Biol 8:27
Ley D, Romantsik O, Vallius S, Sveinsdóttir K, Sveinsdóttir S, Agyemang AA, Baumgarten M, Mörgelin M, Lutay N, Bruschettini M, Holmqvist B, Gram M (2016) High presence of extracellular hemoglobin in the periventricular white matter following preterm intraventricular hemorrhage. Front Physiol 7:330
Tortora D, Severino M, Sedlacik J, Toselli B, Malova M, Parodi A, Morana G, Fato MM, Ramenghi LA, Rossi A (2018) Quantitative susceptibility map analysis in preterm neonates with germinal matrix-intraventricular hemorrhage. J Magn Reson Imaging 48:1199–1207
Ramenghi LA, Fumagalli M, Groppo M, Consonni D, Gatti L, Bertazzi PA, Mannucci PM, Mosca F (2011) Germinal matrix hemorrhage: intraventricular hemorrhage in very-low-birth-weight infants: the independent role of inherited thrombophilia. Stroke 42:1889–1893
Bouyssi-Kobar M, Murnick J, Brossard-Racine M et al (2018) Altered cerebral perfusion in infants born preterm compared with infants born full term. J Pediatr 193:54–61.e2
Jha SC, Xia K, Ahn M, Girault JB, Li G, Wang L, Shen D, Zou F, Zhu H, Styner M, Gilmore JH, Knickmeyer RC (2019) Environmental influences on infant cortical thickness and surface area. Cereb Cortex 29:1139–1149
Jandó G, Mikó-Baráth E, Markó K et al (2012) Early-onset binocularity in preterm infants reveals experience-dependent visual development in humans. Proc Natl Acad Sci U S A 109:11049–11052
Ouyang M, Liu P, Jeon T, Chalak L, Heyne R, Rollins NK, Licht DJ, Detre JA, Roberts TPL, Lu H, Huang H (2017) Heterogeneous increases of regional cerebral blood flow during preterm brain development: preliminary assessment with pseudo-continuous arterial spin labeled perfusion MRI. Neuroimage 147:233–242
Brew N, Walker D, Wong FY (2014) Cerebral vascular regulation and brain injury in preterm infants. Am J Physiol Regul Integr Comp Physiol 306:R773–R786
van Wezel-Meijler G, De Bruïne FT, Steggerda SJ et al (2011) Ultrasound detection of white matter injury in very preterm neonates: practical implications. Dev Med Child Neurol 53(Suppl 4):29–34
Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJP, Wang DJJ, Wong EC, Zaharchuk G (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73:102–116
Ment LR, Duncan CC, Ehrenkranz RA, Lange RC, Taylor KJ, Kleinman CS, Scott DT, Sivo J, Gettner P (1984) Intraventricular hemorrhage in the preterm neonate: timing and cerebral blood flow changes. J Pediatr 104:419–425
Volpe JJ (1986) Positron emission tomography in the newborn: regional CBF in the preterm infant with intraventricular haemorrhage and haemorrhagic intracerebral involvement and in the asphyxiated term infant. In: Rolfe P. Neonatal Physiological Measurements pp 77–85
Kluckow M (2000) Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch Dis Child Fetal Neonatal Ed 82:188F–1194F
Acknowledgments
This work was supported by Eu-Brain Non-profit Association, Genoa, Italy.
Funding
This work was supported by funds from “Ricerca Corrente Disordini Neurologici e Muscolari (Linea 5)” of the Italian Ministry of Health.
Author information
Authors and Affiliations
Contributions
- Domenico Tortora: design of the study, data collection, data analysis, manuscript draft, revision of the manuscript
- Francesco Maria Lo Russo: data analysis, images preparation, revision of the manuscript
- Mariasavina Severino: manuscript draft, data collection, revision of the manuscript
- Alessandro Parodi: data collection, revision of the manuscript
- Paolo Massirio: data collection, revision of the manuscript
- Luca Antonio Ramenghi: data collection, revision of the manuscript
- Andrea Rossi: manuscript draft, revision of the manuscript, design of the study
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Our institutional review (Comitato Etico Regione Liguria) board approved this retrospective study.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Consent to participate
Parents provided informed consent to participate to this study.
Consent for publication
Parents provided informed consent for publication of this study
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tortora, D., Lo Russo, F.M., Severino, M. et al. Regional impairment of cortical and deep gray matter perfusion in preterm neonates with low-grade germinal matrix-intraventricular hemorrhage: an ASL study. Neuroradiology 62, 1689–1699 (2020). https://doi.org/10.1007/s00234-020-02514-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02514-9