Abstract
Purpose
Machado-Joseph disease (MJD/SCA3) is the most frequent spinocerebellar ataxia worldwide. Pathological studies revealed less melanin-containing dopaminergic neurons in the substantia nigra (SN) pars compacta in MJD/SCA3 patients. The purpose of this study was to quantify the damage in the SN reported for MJD/SCA3 patients in vivo using neuromelanin MR imaging.
Methods
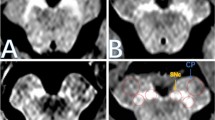
We retrospectively reviewed the clinical information and MR imaging in sixteen MJD/SCA3 patients and fourteen healthy controls. High-resolution T1-WI of the SN were acquired using a 3-T MR system. The neuromelanin-related contrast (NRC), which was defined as the number of pixels of high-signal-intensity areas on T1-WI in the SN, was calculated semi-automatically. The NRC values were compared between MJD/SCA3 patients and normal controls.
Results
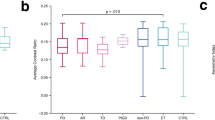
The NRC values were significantly lower in MJD/SCA3 patients than in healthy controls. In MJD/SCA3 patients, a significant negative correlation existed between disease durations and NRC values. No significant difference in the NRC values was revealed between the MJD/SCA3 patients with and without parkinsonism.
Conclusion
Neuromelanin in the SN may decrease at the early stage of the disease and continue to decrease over time with the disease duration in MJD/SCA3 patients. The NRC may be a useful biological index for monitoring MJD/SCA3.




Similar content being viewed by others
Abbreviations
- SCA:
-
Spinocerebellar ataxia
- MJD:
-
Machado-Joseph disease
- CAG repeat:
-
Cytosine-adenine-guanine repeat
- SN:
-
Substantia nigra
- MR:
-
Magnetic resonance
- 3T:
-
3-tesla
- T1-WI:
-
T1-weighted imaging
- NRC:
-
Neuromelanin-related contrast
- DAT:
-
Dopamine transporter
References
Mendonça N, França MC Jr, Gonçalves AF, Januário C (2018) Clinical features of Machado-Joseph disease. Adv Exp Med Biol 1049:255–273
Schöls L, Bauer P, Schmidt T, Schulte T, Riess O (2004) Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol 3:291–304
Kawaguchi Y, Okamoto T, Taniwaki M, Aizawa M, Inoue M, Katayama S, Kawakami H, Nakamura S, Nishimura M, Akiguchi I, Kimura J, Narumiya S, Kakizuka A (1994) CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet 8:221–228
Dürr A, Stevanin G, Cancel G, Duyckaerts C, Abbas N, Didierjean O et al (1996) Spinocerebellar ataxia 3 and Machado-Joseph disease: clinical, molecular, and neuropathological features. Ann Neurol 39:490–499
Schöls L, Reimold M, Seidel K, Globas C, Brockmann K, Hauser TK et al (2015) No parkinsonism in SCA2 and SCA3 despite severe neurodegeneration of the dopaminergic substantia nigra. Brain. 138:3316–3326
Koeppen AH (2018) The neuropathology of spinocerebellar ataxia type 3/Machado-Joseph disease. Adv Exp Med Biol 1049:233–241
Sasaki M, Shibata E, Tohyama K, Takahashi J, Otsuka K, Tsuchiya K, Takahashi S, Ehara S, Terayama Y, Sakai A (2006) Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson’s disease. Neuroreport. 17:1215–1218
Sasaki M, Shibata E, Tohyama K, Kudo K, Endoh J, Otsuka K, Sakai A (2008) Monoamine neurons in the human brain stem: anatomy, magnetic resonance imaging findings, and clinical implications. Neuroreport. 19:1649–1654
Matsuura K, Maeda M, Tabei KI, Umino M, Kajikawa H, Satoh M, Kida H, Tomimoto H (2016) A longitudinal study of neuromelanin-sensitive magnetic resonance imaging in Parkinson’s disease. Neurosci Lett 633:112–117
Kitao S, Matsusue E, Fujii S, Miyoshi F, Kaminou T, Kato S, Ito H, Ogawa T (2013) Correlation between pathology and neuromelanin MR imaging in Parkinson’s disease and dementia with Lewy bodies. Neuroradiology. 55:947–953
Tanaka M, Aihara Y, Ikeda S, Aihara Y (2011) Neuromelanin-related contrast in the substantia nigra semiquantitatively evaluated by magnetic resonance imaging at 3T: comparison between normal aging and Parkinson disease. Rinsho Shinkeigaku 51:14–20 Japanese
Xing Y, Sapuan A, Dineen RA, Auer DP (2018) Life span pigmentation changes of the cubstantia nigra detected by neuromelanin-sensitive MRI. Mor Disord 33:1792–1799
Kawaguchi H, Shimada H, Kodaka F, Suzuki M, Shinotoh H, Hirano S, Kershaw J, Inoue Y, Nakamura M, Sasai T, Kobayashi M, Suhara T, Ito H (2016) Principal component analysis of multimodal neuromelanin MRI and dopamine transporter PET data provides a specific metric for the nigral dopaminergic neuronal density. PLoS One 11:e0151191
Rezende TJR, de Paiva JLR, Martinez ARM, Lopes-Cendes I, Pedroso JL, Barsottini OGP, Cendes F, França MC Jr (2018) Structural signature of SCA3: from presymptomatic to late disease stages. Ann Neurol 84:401–408
Lukas C, Schöls L, Bellenberg B, Rüb U, Przuntek H, Schmid G, Köster O, Suchan B (2006) Dissociation of grey and white matter reduction in spinocerebellar ataxia type 3 and 6: a voxel-based morphometry study. Neurosci Lett 408:230–235
D’Abreu A, França M Jr, Appenzeller S, Lopes-Cendes I, Cendes F (2009) Axonal dysfunction in the deep white matter in Machado-Joseph disease. J Neuroimaging 19:9–12
Horimoto Y, Matsumoto M, Akatsu H, Kojima A, Yoshida M, Nokura K, Yuasa H, Katada E, Yamamoto T, Kosaka K, Hashizume Y, Yamamoto H, Mitake S (2011) Longitudinal study on MRI intensity changes of Machado-Joseph disease: correlation between MRI findings and neuropathological changes. J Neurol 258:1657–1664
de Oliveira MS, D’Abreu A, França MC Jr, Lopes-Cendes I, Cendes F, Castellano G (2012) MRI-texture analysis of corpus callosum, thalamus, putamen, and caudate in Machado-Joseph disease. J Neuroimaging 22:46–52
Eichler L, Bellenberg B, Hahn HK, Köster O, Schöls L, Lukas C (2011) Quantitative assessment of brain stem and cerebellar atrophy in spinocerebellar ataxia types 3 and 6: impact on clinical status. AJNR Am J Neuroradiol 32:890–897
Camargos ST, Marques W Jr, Santos AC (2011) Brain stem and cerebellum volumetric analysis of Machado Joseph disease patients. Arq Neuropsiquiatr 69:292–296
D’Abreu A, França MC Jr, Yasuda CL, Campos BA, Lopes-Cendes I, Cendes F (2012) Neocortical atrophy in Machado-Joseph disease: a longitudinal neuroimaging study. J Neuroimaging 22:285–291
Guimarães RP, D’Abreu A, Yasuda CL, França MC Jr, Silva BH, Cappabianco FA et al (2013) A multimodal evaluation of microstructural white matter damage in spinocerebellar ataxia type 3. Mov Disord 28:1125–1132
Lopes TM, D’Abreu A, França MC Jr, Yasuda CL, Betting LE, Samara AB et al (2013) Widespread neuronal damage and cognitive dysfunction in spinocerebellar ataxia type 3. J Neurol 260:2370–2379
Kang JS, Klein JC, Baudrexel S, Deichmann R, Nolte D, Hilker R (2014) White matter damage is related to ataxia severity in SCA3. J Neurol 261:291–299
Xing W, Wang XY, Liao XX, Liao WH, Shen L (2014) Spin labeling artery method perfusion MRI study of SPG4 and SCA3/MJD. Magn Reson Imaging 32:1330–1334
de Rezende TJ, D’Abreu A, Guimarães RP, Lopes TM, Lopes-Cendes I, Cendes F et al (2015) Cerebral cortex involvement in Machado-Joseph disease. Eur J Neurol 22:277–283
Stefanescu MR, Dohnalek M, Maderwald S, Thürling M, Minnerop M, Beck A, Schlamann M, Diedrichsen J, Ladd ME, Timmann D (2015) Structural and functional MRI abnormalities of cerebellar cortex and nuclei in SCA3, SCA6 and Friedreich’s ataxia. Brain. 138:1182–1197
Wang TY, Jao CW, Soong BW, Wu HM, Shyu KK, Wang PS, Wu YT (2015) Change in the cortical complexity of spinocerebellar ataxia type 3 appears earlier than clinical symptoms. PLoS One 10:e0118828
Duarte JV, Faustino R, Lobo M, Cunha G, Nunes C, Ferreira C, Januário C, Castelo-Branco M (2016) Parametric fMRI of paced motor responses uncovers novel whole-brain imaging biomarkers in spinocerebellar ataxia type 3. Hum Brain Mapp 37:3656–3668
Huang SR, Wu YT, Jao CW, Soong BW, Lirng JF, Wu HM et al (2016) CAG repeat length does not associate with the rate of cerebellar degeneration in spinocerebellar ataxia type 3. Neuroimage Clin 13:97–105
Hernandez-Castillo CR, Diaz R, Campos-Romo A, Fernandez-Ruiz J (2017) Neural correlates of ataxia severity in spinocerebellar ataxia type 3/Machado-Joseph disease. Cerebellum Ataxias 4:7
Meles SK, Kok JG, De Jong BM, Renken RJ, de Vries JJ, Spikman JM et al (2018) The cerebral metabolic topography of spinocerebellar ataxia type 3. Neuroimage Clin 19:90–97
Peng H, Liang X, Long Z, Chen Z, Shi Y, Xia K, Meng L, Tang B, Qiu R, Jiang H (2019) Gene-related cerebellar neurodegeneration in SCA3/MJD: a case-controlled imaging-genetic study. Front Neurol 10:1025
Yen TC, Lu CS, Tzen KY, Wey SP, Chou YH, Weng YH, Kao PF, Ting G (2000) Decreased dopamine transporter binding in Machado-Joseph disease. J Nucl Med 41:994–998
Ogisu K, Kudo K, Sasaki M, Sakushima K, Yabe I, Sasaki H, Terae S, Nakanishi M, Shirato H (2013) 3D neuromelanin-sensitive magnetic resonance imaging with semi-automated volume measurement of the substantia nigra pars compacta for diagnosis of Parkinson’s disease. Neuroradiology. 55:719–724
Castellanos G, Fernández-Seara MA, Lorenzo-Betancor O, Ortega-Cubero S, Puigvert M, Uranga J, Vidorreta M, Irigoyen J, Lorenzo E, Muñoz-Barrutia A, Ortiz-de-Solorzano C, Pastor P, Pastor MA (2015) Automated neuromelanin imaging as a diagnostic biomarker for Parkinson’s disease. Mov Disord 30:945–952
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The present study was retrospective. Our local ethics committee did not require the study’s approval or the patients’ informed consent for this retrospective review of patients’ records and images except for the patients’ genetic information. In addition, our local ethics committee approved using genetic information for this research.
The healthy volunteers were recruited and examined at our hospital. Our local ethics committee approved the MR study of healthy volunteers for research.
Informed consent
Written informed consent was obtained from all patients and all healthy volunteers.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakata, Y., Sakamoto, A. & Kawata, A. Neuromelanin imaging analyses of the substantia nigra in patients with Machado-Joseph disease. Neuroradiology 62, 1433–1439 (2020). https://doi.org/10.1007/s00234-020-02479-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02479-9