Abstract
Purpose
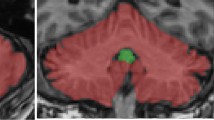
To assess the prevalence and characteristics of motor cortex hypointensity on 3-T susceptibility-weighted imaging (SWI) in patients with cognitive impairment and examine its clinical significance.
Methods
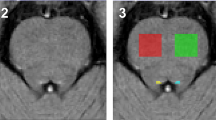
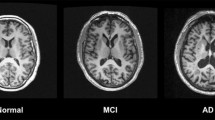
The institutional review board approved this retrospective study and waived the requirement for informed consent. A total of 127 patients with a clinical diagnosis of probable Alzheimer’s disease (AD) (n = 32) or mild cognitive impairment (MCI) (n = 95) and 127 age- and sex-matched control subjects underwent 3-T brain magnetic resonance imaging. SWI was analyzed for both subjective visual scoring and the quantitative estimation of phase shift in the posterior bank of the motor cortex. A multivariate logistic regression analysis was performed to identify clinical and imaging variables associated with motor cortex hypointensity on SWI.
Results
Motor cortex hypointensity on SWI was observed in 94/127 cognitively impaired patients (74.0%) and 72/127 control subjects (56.7%) (p = 0.004). Age was the only variable that was significantly associated with motor cortex hypointensity in patients with cognitive impairment (odds ratio, 1.15; 95% confidence interval, 1.065–1.242; p < 0.001). The quantitative analysis confirmed a significant increase in phase shifting in the posterior bank of the motor cortex in patients with positive motor cortex hypointensity on SWI (p < 0.001).
Conclusion
Motor cortex hypointensity on SWI was more frequently found in patients with cognitive impairment than in age-matched controls and was positively associated with age. Thus, it may be a potential imaging marker of iron accumulation in patients with MCI or AD.



Similar content being viewed by others
References
Hirai T, Korogi Y, Sakamoto Y, Hamatake S, Ikushima I, Takahashi M (1996) T2 shortening in the motor cortex: effect of aging and cerebrovascular diseases. Radiology 199:799–803
Imon Y, Yamaguchi S, Yamamura Y, Tsuji S, Kajima T, Ito K, Nakamura S (1995) Low intensity areas observed on T2-weighted magnetic resonance imaging of the cerebral cortex in various neurological diseases. J Neurol Sci 134(Suppl):27–32
Iwasaki Y, Ikeda K, Shiojima T, Tagaya M, Kurihara T, Kinoshita M (1994) Clinical significance of hypointensity in the motor cortex on T2-weighted images. Neurology 44:1181
Ngai S, Tang YM, Du L, Stuckey S (2007) Hyperintensity of the precentral gyral subcortical white matter and hypointensity of the precentral gyrus on fluid-attenuated inversion recovery: variation with age and implications for the diagnosis of amyotrophic lateral sclerosis. AJNR Am J Neuroradiol 28:250–254
Kamada K, Kakeda S, Ohnari N, Moriya J, Sato T, Korogi Y (2008) Signal intensity of motor and sensory cortices on T2-weighted and flair images: intraindividual comparison of 1.5T and 3T MRI. Eur Radiol 18:2949–2955
Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC (2009) Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol 30:19–30
Adachi Y, Sato N, Saito Y, Kimura Y, Nakata Y, Ito K, Kamiya K, Matsuda H, Tsukamoto T, Ogawa M (2015) Usefulness of SWI for the detection of iron in the motor cortex in amyotrophic lateral sclerosis. J Neuroimaging 25:443–451
Kakeda S, Yoneda T, Ide S, Miyata M, Hashimoto T, Futatsuya K, Watanabe K, Ogasawara A, Moriya J, Sato T, Okada K, Uozumi T, Adachi H, Korogi Y (2016) Zebra sign of precentral gyri in amyotrophic lateral sclerosis: a novel finding using phase difference enhanced (padre) imaging-initial results. Eur Radiol 26:4173–4183
Sheelakumari R, Madhusoodanan M, Radhakrishnan A, Ranjith G, Thomas B (2016) A potential biomarker in amyotrophic lateral sclerosis: can assessment of brain iron deposition with SWI and corticospinal tract degeneration with DTI help? AJNR Am J Neuroradiol 37:252–258
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999) Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56:303–308
Costagli M, Donatelli G, Biagi L, Caldarazzo Ienco E, Siciliano G, Tosetti M, Cosottini M (2016) Magnetic susceptibility in the deep layers of the primary motor cortex in amyotrophic lateral sclerosis. NeuroImage: Clinical 12:965–969
van Rooden S, Versluis MJ, Liem MK, Milles J, Maier AB, Oleksik AM, Webb AG, van Buchem MA, van der Grond J (2014) Cortical phase changes in Alzheimer’s disease at 7T MRI: a novel imaging marker. Alzheimers Dement 10:e19–e26
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149:351–356
Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge Rv, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M, STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1) (2013) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12:822–838
Cosottini M, Donatelli G, Costagli M, Ienco EC, Frosini D, Pesaresi I, Biagi L, Siciliano G, Tosetti M (2016) High-resolution 7T MR imaging of the motor cortex in amyotrophic lateral sclerosis. Am J Neuroradiol 37:455–461
Kwan JY, Jeong SY, Van Gelderen P et al (2012) Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 tesla MRI and pathology. PLoS One 7:e35241
Schweitzer AD, Liu T, Gupta A, Zheng K, Seedial S, Shtilbans A, Shahbazi M, Lange D, Wang Y, Tsiouris AJ (2015) Quantitative susceptibility mapping of the motor cortex in amyotrophic lateral sclerosis and primary lateral sclerosis. AJR Am J Roentgenol 204:1086–1092
Drayer BP (1988) Imaging of the aging brain. Part II. Pathologic conditions. Radiology 166:797–806
Hecht MJ, Fellner F, Fellner C, Hilz MJ, Neundorfer B, Heuss D (2002) Hyperintense and hypointense MRI signals of the precentral gyrus and corticospinal tract in ALS: a follow-up examination including flair images. J Neurol Sci 199:59–65
Petri S, Korner S, Kiaei M (2012) Nrf2/ARE signaling pathway: key mediator in oxidative stress and potential therapeutic target in ALS. Neurol Res Int 2012:878030
Moon Y, Han SH, Moon WJ (2016) Patterns of brain iron accumulation in vascular dementia and Alzheimer’s dementia using quantitative susceptibility mapping imaging. J Alzheimers Dis 51:737–745
Park M, Moon WJ, Moon Y, Choi JW, Han SH, Wang Y (2018) Region-specific susceptibility change in cognitively impaired patients with diabetes mellitus. PLoS One 13:e0205797
Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L (2014) The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13:1045–1060
Bulk M, Abdelmoula WM, Nabuurs RJA, van der Graaf LM, Mulders CWH, Mulder AA, Jost CR, Koster AJ, van Buchem MA, Natté R, Dijkstra J, van der Weerd L (2018) Postmortem MRI and histology demonstrate differential iron accumulation and cortical myelin organization in early- and late-onset Alzheimer’s disease. Neurobiol Aging 62:231–242
van Duijn S, Bulk M, van Duinen SG, Nabuurs RJA, van Buchem MA, van der Weerd L, Natte R (2017) Cortical iron reflects severity of Alzheimer’s disease. J Alzheimers Dis 60:1533–1545
van Rooden S, Doan NT, Versluis MJ, Goos JDC, Webb AG, Oleksik AM, van der Flier WM, Scheltens P, Barkhof F, Weverling–Rynsburger AWE, Blauw GJ, Reiber JHC, van Buchem MA, Milles J, van der Grond J (2015) 7T T(2)*-weighted magnetic resonance imaging reveals cortical phase differences between early- and late-onset Alzheimer’s disease. Neurobiol Aging 36:20–26
Hallgren B, Sourander P (1958) The effect of age on the non-haemin iron in the human brain. J Neurochem 3:41–51
Reichenbach JR, Schweser F, Serres B, Deistung A (2015) Quantitative susceptibility mapping: concepts and applications. Clin Neuroradiol 25(Suppl 2):225–230
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was funded by the National Research Foundation of Korea (NRF) Grant funded by the Korean government (MSIP) (No. 2017R1A2B4010634) and by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHID), funded by the Ministry of Health and Welfare, Republic of Korea (Grant No. HI18C1038).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
For this type of retrospective study formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, M., Moon, Y., Han, SH. et al. Motor cortex hypointensity on susceptibility-weighted imaging: a potential imaging marker of iron accumulation in patients with cognitive impairment. Neuroradiology 61, 675–683 (2019). https://doi.org/10.1007/s00234-019-02159-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-019-02159-3