Abstract
Purpose
To describe the neuroradiological features of intraocular medulloepithelioma.
Methods
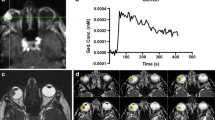
We retrospectively analyzed the clinical, histopathological, and MRI data of five children with medulloepithelioma. In addition to conventional images, DWI was performed in four patients and mean ADC was calculated; this was limited to the technique of this cohort of patients. DCE was performed in all patients. This is the first paper that presents diffusion and perfusion characteristics of medulloepithelioma.
Results
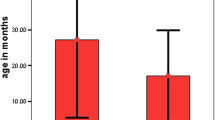
Four tumors were malignant teratoid variants, two non-teratoid variants. Tumors were hyperintense on T1-weighted images and hypointense on T2-weighted images. Calcifications were detectable in two out of five tumors. Cavities were detectable in three out of five tumors. All tumors showed some degree of enhancement. The mean ADC of all four patients was 1.156 ± 242.75 × 10−3 mm2/s. Mean ktrans, Ve, Kep, TME, AUC, SER, and peak enhancement were 0.082 ± 0.054, 0.19 ± 0.076, 0.31 ± 0.084, 0.97 ± 0.0784, 1.22 ± 0.81, 67.34 ± 31.7, and 14.84 ± 7.34 respectively. TICs showed a very high ratio of slow increase, > 50% persistence and some degree of wash out. Teratoid variants showed higher K-trans, AUC, VE, TME, and persistent TIC pattern than non-teratoid ones, while plateau pattern ratio was lower.
Conclusion
Conventional MR findings were similar to previously reported cases. Mean ADCs were moderately high. TICs showed slow increase and presence of wash out. K-trans, AUC, VE, and TME were higher in teratoid variants. Permeability parameters in differential diagnosis with lesions mimicking medulloepithelioma need further investigations.






Similar content being viewed by others
References
Saunders T, Margo CE (2012) Intraocular medulloepithelioma. Arch Pathol Lab Med 136:212–216
Zimmerman LE (1971) Verhoeff’s “terato-neuroma.” A critical reappraisal in light of new observations and current concepts of embryonic tumors. Am J Ophthalmol 72:1039–1057
Cerase A, De Francesco S, Citterio A, Hadjistilianou T, Malandrini A, Mastrangelo D, Toti P, Venturi C (2010) Growth of congenital malignant teratoid medulloepithelioma of the ciliary body: a case study. J Neuro-Oncol 96:443–448
McLendon RE, Judkins AR, Eberhart CG, Fuller GN, Sarkar C, Ng HK (2007) Central nervous system primitive neuroectodermal tumours. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds) WHO classification of tumours of the nervous system. IARC, Lyon, pp 141–146
Pushker N, Khuraijam N, Sen S, Bajaj M, Mehta M (2008) Medulloepithelioma of the ciliary body associated with massive intravitreal hemorrhage in an adult. Can J Ophthalmol 43:253–254
Broughton WL, Zimmerman LE (1978) A clinicopathologic study of 56 cases of intraocular medulloepitheliomas. Am J Ophthalmol 85:407–418
Shields JA, Shields CL (1999) Miscellaneous intraocular tumors. In: Shields JA, Shields CL (eds) Atlas of intraocular tumors. Lippincott, Williams & Wilkins, Philadelphia, pp 310–315
Shields JA, Eagle RC, Shields CL et al (1996) Congenital neoplasms of the non pigmented ciliary epithelium (medulloepithelioma). Ophthalmology 103(12):1998–2006
Husain S, Husain N, Boniuk M, Font RL (1998) Malignant nonteratoid medulloepithelioma of the ciliary body in an adult. Ophthalmology 105:596–599
Sosinska-Mielcarek K, Senkus-Konefka E, Jaskiewicz K, Kordek R, Jassem J (2006) Intraocular malignant teratoid medulloepithelioma in an adult: clinicopathological case report and review of the literature. Acta Ophthalmol Scand 84:259–262
Zimmerman LE, Broughton WL (1978) A clinicopathologic and follow-up study of fifty-six intraocular medulloepitheliomas. In: Jackobiec FA (ed) Ocular and adnexal tumors. Aesculapius Publishing, Birmingham, pp 181–196
Sansgiri RK, Wilson M, Carville MB, Helton KJ (2013) Imaging features of medulloepithelioma: report of four cases and review of the literature. Pediatr Radiol 43:1344–1356
Karcioglu ZA, Abboud EB, Al-Mesfer SA et al (2002) Retinoblastoma in older children. JAAPOS 6(1):26–32
Mamalsi N, Font RL, Anderson CW et al (1992) Concurrent benign teratoid medulloepithelioma and pineoblastoma. Ophthalmic Surg 23(6):403–408
Steinkuller PG, Font RL (1997) Congenital malignant teratoid neoplasms of the eye and orbit: a case report and review of the literature. Ophthalmology 104(1):38–42
Yanko L, Behar A (1978) Teratoid intraocular medulloepithelioma. Am J Ophthalmol 85:850–853
Carrillo R, Streeten BW (1979) Malignant teratoid medulloepithelioma in an adult. Arch Ophthalmol 97:695–699
Verdijk R (2016) On the classification and grading of medulloepithelioma of the eye. Ocular Oncology and Pathology 2:190–193
Galluzzi P, Cerase A, Hadjistilianou T, de Francesco S, Toti P, Vallone IM, Filosomi G, Monti L, Bracco S, Gennari P, Ginanneschi C, Venturi C (2003) Retinoblastoma: abnormal gadolinium-enhancement of the anterior segment of eyes at MR imaging with clinical and pathological correlation. Radiology 228:683–690
Galluzzi P, Hadjistilianou T, Cerase A, De Francesco S, Toti P, Venturi C (2009) Is CT still useful in the study protocol of retinoblastoma? AJNR Am J Neuroradiol 30:1760–1765
Rodjan F, de Graaf P, van der Valk P, Hadjistilianou T, Cerase A, Toti P, de Jong PC, Moll AC, Castelijns AJ, Galluzzi P, on behalf of the European Retinoblastoma Imaging Collaboration (2015) Detection of calcifications in retinoblastoma using gradient-echo MR imaging sequences: comparative study between in vivo MR imaging and ex vivo high-resolution CT. AJNR Am J Neuroradiol 36:355–360
Abdel Razek AA et al (2011) Differentiation between benign and malignant orbital tumors at 3-T diffusion MR imaging. Neuroradiology 53(7):517–522
Sepahdari AR, Aakalu VK, Setabutr P, Shiehmorteza M, Naheedy JH, Mafee MF (2010) Indeterminate orbital masses: restricted diffusion at MR imaging with echo-planar diffusion-weighted imaging predicts malignancy. Radiology 256(2):554–564
Abdel Razek AA et al (2012) Correlation of apparent diffusion coefficient at 3T with prognostic parameters of retinoblastoma. Am J Neuroradiol 33(5):944–948
de Graaf P, Pouwels PJW, Rodjan F, Moll AC, Imhof SM, Knol DL, Sanchez E, van der Valk P, Castelijns JA (2012) Single-shot turbo spin-echo diffusion-weighted imaging for retinoblastoma: initial experience. Am J Neuroradiol 33(1):110–118
Cui Y, Luo R, Wang R, Liu H, Zhang C, Zhang Z, Wang D (2018) Correlation between conventional MR imaging combined with diffusion-weighted imaging and histopathologic findings in eyes primarily enucleated for advanced retinoblastoma: a retrospective study. Eur Radiol 28(2):620–629
Chen S, Ji X, Liu M, Xia Z, Zheng H, Yin Q, Wang H, Li Y (2017) The value of MRI in evaluating the efficacy and complications with the treatment of intra-arterial chemotherapy for retinoblastoma. Oncotarget 8(24):38413–38425
Sepahdari AR, Kapur R, Aakalu VK, Villablanca JP, Mafee MF (2012) Diffusion-weighted imaging of malignant ocular masses: initial results and directions for further study. AJNR Am J Neuroradiol 33:314–319
Galluzzi P (2018) Potential value of apparent diffusion coefficient (ADC) in first-line treatment of retinoblastoma. EC Ophthalmology 3:109–118
Xian J, Zhang Z, Wang Z, Li J, Yang B, Man F, Chang Q, Zhang Y (2010) Value of MR imaging in the differentiation of benign and malignant orbital tumors in adults. Eur Radiol 20(7):1692–1702
Xu XQ, Qian W, Ma G, Hu H, Su GY, Liu H, Shi HB, Wu FY (2017) Combined diffusion-weighted imaging and dynamic contrast-enhanced MRI for differentiating radiologically indeterminate malignant from benign orbital masses. Clin Radiol 72(10):903.e9–903.90
Ro SR, Asbach P, Siebert E et al (2016) Characterization of orbital masses by multiparametric MRI. Eur J Radiol 85(2):324–336
Sun B, Song L, Wang X, Li J, Xian J, Wang F, Tan P (2017) Lymphoma and inflammation in the orbit: diagnostic performance with diffusion-weighted imaging and dynamic contrast-enhanced MRI. J Magn Reson Imaging 45:1438–1445
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Galluzzi, P., Casseri, T., Cerase, A. et al. Conventional, diffusion, and permeability MR findings in ocular medulloepithelioma. Neuroradiology 60, 1213–1222 (2018). https://doi.org/10.1007/s00234-018-2094-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-018-2094-1