Abstract
Purpose
We investigated the correlation between tumor blood flow (TBF) and histopathologic features of Warthin tumors (WTs) and pleomorphic adenomas (PAs) to determine the TBF in the differential diagnosis of these tumors and evaluated how well pCASL-MRI can differentiate PAs from WTs compared to conventional MRI.
Methods
The ADC, time intensity curve (TIC) pattern of dynamic contrast enhancement, and pCASL (visual assessment and TBF of the MR images of 10 WTs and 13 PAs) were reviewed. We compared the pCASL and ADC or TIC patterns in WT and PA images. Tissue sections were stained with CD34 to evaluate microvessel density (MVD). The TBF and MVD results were compared. The Mann-Whitney U test was used to compare the TBFs, ADCs, and MVDs of these tumors. The diagnostic accuracy was determined by analyzing the receiver operating characteristic curve.
Results
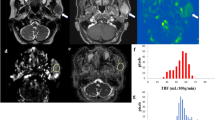
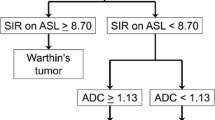
On visual assessment, the signal intensity was higher in all but three cases of WT. The TBF and MVD of the WTs were significantly higher (both, p < 0.01) than the PAs, and the ADC was significantly lower (p < 0.01). Many WTs had early enhancement of the TIC pattern and high washout; many PAs had gradual enhancement. The diagnostic accuracies of visual analysis, TBF, and ADC for differentiation between WTs and PAs were 91.3, 95.7, and 87.0%, respectively.
Conclusions
The TBF were significantly higher in WTs than in PAs, and there was a positive correlation between TBF and MVD. Moreover, pCASL-MRI provides more accurate imaging than conventional MRI to differentiate WTs and PAs.







Similar content being viewed by others
Abbreviations
- ADC:
-
apparent diffusion coefficiency
- ASL:
-
arterial spin labeling
- AUC:
-
area under the curve
- CBF:
-
cerebral blood flow
- CNS:
-
central nervous system
- DWI:
-
diffusion weighted image
- EPI:
-
echo-planar imaging
- FOV:
-
field of view
- FSE:
-
fast spin-echo
- MRI:
-
magnetic resonance imaging
- MVD:
-
microvessel density
- NEX:
-
number of excitation
- NPV:
-
negative predictive value
- NRI:
-
net reclassification improvement
- PA:
-
pleomorphic adenoma
- PASL:
-
pulsed ASL
- pCASL:
-
pseudo-continuous ASL
- PPV:
-
positive predictive value
- ROC:
-
receiver operating characteristic
- ROI:
-
region of interest
- SNR:
-
signal-to-noise ratio
- T1WI:
-
T1 weighted image
- T2WI:
-
T2 weighted image
- TBF:
-
tumor blood flow
- TIC:
-
time intensity curve
- TSE:
-
turbo spin-echo
- WR:
-
washout ratio
- WT:
-
Warthin tumor
References
Shimizu M, Ussmuller J, Hartwein J et al (1999) Statistical study for sonographic differential diagnosis of tumorous lesions in the parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 88:226–233
Som PM, Brandwein-Gensler MS (2011) Anatomy and pathology of the salivary gland, epithelial tumors. In: Som PM, Curtin HD (eds) Head and neck imaging, 5th edn. Mosby, St Louis, pp 2525–2544
Wittich GR, Scheible WF, Hajek PC (1985) Ultrasonography of the salivary glands. Radiol Clin N Am 23:29–37
Zajkowski P, Jakubowski W, Białek EJ, Wysocki M, Osmólski A, Serafin-Król M (2000) Pleomorphic adenoma and adenolymphoma in ultrasonography. Eur J Ultrasound 12:23–29
Yabuuchi H, Fukuya T, Tajima T, Hachitanda Y, Tomita K, Koga M (2003) Salivary gland tumors: diagnostic value of gadolinium-enhanced dynamic MR imaging with histopathologic correlation. Radiology 226:345–354
Ikeda M, Motoori K, Hanazawa T, Nagai Y, Yamamoto S, Ueda T, Funatsu H, Ito H (2004) Warthin tumor of the parotid gland: diagnostic value of MR imaging with histopathologic correlation. AJNR Am J Neuroradiol 25:1256–1262
Yerli H, Aydin E, Coskun M, Geyik E, Ozluoglu LN, Haberal N, Kaskati T (2007) Dynamic multislice computed tomography findings for parotid gland tumors. J Comput Assist Tomogr 31:309–316
Hisatomi M, Asaumi J, Yanagi Y, Unetsubo T, Maki Y, Murakami J, Matsuzaki H, Honda Y, Konouchi H (2007) Diagnostic value of dynamic contrast-enhanced MRI in the salivary gland tumors. Oral Oncol 43:940–947
Christe A, Waldherr C, Hallett R, Zbaeren P, Thoeny H (2011) MR imaging of parotid tumors: typical lesion characteristics in MR imaging improve discrimination between benign and malignant disease. AJNR Am J Neuroradiol 32:1202–1207
Yabuuchi H, Matsuo Y, Kamitani T, Setoguchi T, Okafuji T, Soeda H, Sakai S, Hatakenaka M, Nakashima T, Oda Y, Honda H (2008) Parotid gland tumors: can addition of diffusion-weighted MR imaging to dynamic contrast-enhanced MR imaging improve diagnostic accuracy in characterization? Radiology 249:909–916
Eida S, Sumi M, Nakamura T (2010) Multiparametric magnetic resonance imaging for the differentiation between benign and malignant salivary gland tumors. J Magn Reson Imaging 31:673–679
Habermann CR, Arndt C, Graessner J, Diestel L, Petersen KU, Reitmeier F, Ussmueller JO, Adam G, Jaehne M (2009) Diffusion-weighted echo-planar MR imaging of primary parotid gland tumors: is a prediction of different histologic subtypes possible? AJNR Am J Neuroradiol 30:591–596
Eida S, Sumi M, Sakihama N, Takahashi H, Nakamura T (2007) Apparent diffusion coefficient mapping of salivary gland tumors: prediction of the benignancy and malignancy. AJNR Am J Neuroradiol 28:116–121
Matsushima N, Maeda M, Takamura M, Takeda K (2007) Apparent diffusion coefficients of benign and malignant salivary gland tumors. Comparison to histopathological findings. J Neuroradiol 34:183–189
Kimura H, Takeuchi H, Koshimoto Y, Arishima H, Uematsu H, Kawamura Y, Kubota T, Itoh H (2006) Perfusion imaging of meningioma by using continuous arterial spin-labeling: comparison with dynamic susceptibility-weighted contrast-enhanced MR images and histopathologic features. AJNR Am J Neuroradiol 27:85–93
Noguchi T, Yoshiura T, Hiwatashi A, Togao O, Yamashita K, Nagao E, Shono T, Mizoguchi M, Nagata S, Sasaki T, Suzuki SO, Iwaki T, Kobayashi K, Mihara F, Honda H (2008) Perfusion imaging of brain tumors using arterial spin-labeling: correlation with histopathologic vascular density. AJNR Am J Neuroradiol 29:688–693
Weber MA, Thilmann C, Lichy MP et al (2004) Assessment of irradiated brain metastases by means of arterial spin-labeling and dynamic susceptibility-weighted contrast-enhanced perfusion MRI: initial results. Investig Radiol 39:277–287
Yamamoto T, Kinoshita K, Kosaka N, Sato Y, Shioura H, Takeuchi H, Kimura H (2013) Monitoring of extra-axial brain tumor response to radiotherapy using pseudo-continuous arterial spin labeling images: preliminary results. Magn Reson Imaging 31:1271–1277
Shimizu K, Kosaka N, Fujiwara Y, et al (2016) Arterial transit time-corrected renal blood flow measurement with pulsed continuous arterial spin labeling MR imaging. Magn Reson Med Sci 2016 May 9
Yamamoto T, Kosaka N, Mori M, Imamura Y, Kimura H (2014) Assessment of tumor blood flow and its correlation with histopathologic features in Warthin tumors and pleomorphic adenomas of the salivary gland by using pulsed-continuous arterial spin labeling images. In: Proceedings of the Joint Annual Meeting ISMRM-ESMRMB, Milan, Italy
Kato H, Kanematsu M, Watanabe H, Kajita K, Mizuta K, Aoki M, Okuaki T (2015) Perfusion imaging of parotid gland tumours: usefulness of arterial spin labeling for differentiating Warthin's tumours. Eur Radiol 25:3247–3254
Dai W, Garcia D, de Bazelaire C, Alsop DC (2008) Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 60:1488–1497
Alsop DC, Detre JA (1996) Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab 16:1236–1249
Wang J, Zhang Y, Wolf RL, Roc AC, Alsop DC, Detre JA (2005) Amplitude-modulated continuous arterial spin-labeling 3.0-T perfusion MR imaging with a single coil: feasibility study. Radiology 235:218–228
Herscovitch P, Raichle ME (1985) What is the correct value for the brain - blood partition coefficient for water? J Cereb Blood Flow Metab 5:65–69
Garcia DM, Duhamel G, Alsop DC (2005) Efficiency of inversion pulses for background suppressed arterial spin labeling. Magn Reson Med 54:366–372
Bosari S, Lee AK, DeLellis RA et al (1992) Microvessel quantitation and prognosis in invasive breast carcinoma. Hum Pathol 23:755–761
Pepe MS, Fan J, Feng Z, Gerds T, Hilden J (2015) The net reclassification index (NRI): a misleading measure of prediction improvement even with independent test data sets. Stat Biosci 7:282–295
Liu W, Lou X, Ma L (2016) Use of 3D pseudo-continuous arterial spin labeling to characterize sex and age differences in cerebral blood flow. Neuroradiology 58:943–948
Woo SH, Choi DS, Kim JP, Park JJ, Joo YH, Chung PS, Kim BY, Ko YH, Jeong HS, Kim HJ (2013) Two-phase computed tomography study of Warthin tumor of parotid gland: differentiation from other parotid gland tumors and its pathologic explanation. J Comput Assist Tomogr 37:518–524
Yamamoto T, Takeuchi H, Kinoshita K, Kosaka N, Kimura H (2014) Assessment of tumor blood flow and its correlation with histopathologic features in skull base meningiomas and schwannomas by using pseudo-continuous arterial spin labeling images. Eur J Radiol 83:817–823
Motoori K, Ueda T, Uchida Y, Chazono H, Suzuki H, Ito H (2005) Identification of Warthin tumor: magnetic resonance imaging versus salivary scintigraphy with technetium-99m pertechnetate. J Comput Assist Tomogr 29:506–512
Takumi K, Fukukura Y, Hakamada H, Ideue J, Kumagae Y, Yoshiura T (2017) Value of diffusion tensor imaging in differentiating malignant from benign parotid gland tumors. Eur J Radiol 95:249–256
Mikayama R, Yabuuchi H, Sonoda S, Kobayashi K, Nagatomo K, Kimura M, Kawanami S, Kamitani T, Kumazawa S, Honda H (2018) Comparison of intravoxel incoherent motion diffusion-weighted imaging between turbo spin-echo and echo-planar imaging of the head and neck. Eur Radiol 28:316–324
Sigmund EE, Jensen J (2011) Basic physical principles of body diffusion-weighted MRI. In: Taouli B (ed) Extra-cranial applications of diffusion-weighted MRI. Cambridge University Press, Cambridge, pp 1–17
Verhappen MH, Pouwels PJ, Ljumanovic R et al (2012) Diffusion weighted MR imaging in head and neck cancer: comparison between half-fourier acquisition single-shot turbo spin-echo and EPI techniques. AJNR 33:1239–1246
Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJP, Wang DJJ, Wong EC, Zaharchuk G (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73:102–116
Guerra G, Testa D, Montagnani S, Tafuri D, Salzano FA, Rocca A, Amato B, Salzano G, Dell'Aversana Orabona G, Piombino P, Motta G (2014) Surgical management of pleomorphic adenoma of parotid gland in elderly patients: role of morphological features. Int J Surg 12(Suppl 2):S12–S16
Ananthaneni A, Undavalli SB (2013) Juvenile cellular pleomorphic adenoma. BMJ Case Rep 2013:bcr2012007641. https://doi.org/10.1136/bcr-2012-007641
McGregor AD, Burgoyne M, Tan KC (1988) Recurrent pleomorphic salivary adenoma-the relevance of age at first presentation. Br J Plast Surg 41:177–181
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded in part by Grants-in-Aid for Scientific Research (C) (15k09916) from the Japan Society for the Promotion of Science (HK).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Ethical standards
We declare that all human and animal studies have been approved by the University of Fukui Ethics Committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. For this type of study formal consent is not required.
Informed consent
For this type of retrospective study formal consent is not required.
Rights and permissions
About this article
Cite this article
Yamamoto, T., Kimura, H., Hayashi, K. et al. Pseudo-continuous arterial spin labeling MR images in Warthin tumors and pleomorphic adenomas of the parotid gland: qualitative and quantitative analyses and their correlation with histopathologic and DWI and dynamic contrast enhanced MRI findings. Neuroradiology 60, 803–812 (2018). https://doi.org/10.1007/s00234-018-2046-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-018-2046-9