Abstract
Purpose
Despite complex olfactory bulb embryogenesis, its development abnormalities in tuberous sclerosis complex (TSC) have been poorly investigated.
Methods
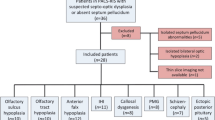
Brain MRIs of 110 TSC patients (mean age 11.5 years; age range 0.5–38 years; 52 female; 26 TSC1, 68 TSC2, 8 without mutation identified in TSC1 or TSC2, 8 not tested) were retrospectively evaluated. Signal and morphological abnormalities consistent with olfactory bulb hypo/aplasia or with olfactory bulb hamartomas were recorded. Cortical tuber number was visually assessed and a neurological severity score was obtained. Patients with and without rhinencephalon abnormalities were compared using appropriate parametric and non-parametric tests.
Results
Eight of110 (7.2%) TSC patients presented rhinencephalon MRI changes encompassing olfactory bulb bilateral aplasia (2/110), bilateral hypoplasia (2/110), unilateral hypoplasia (1/110), unilateral hamartoma (2/110), and bilateral hamartomas (1/110); olfactory bulb hypo/aplasia always displayed ipsilateral olfactory sulcus hypoplasia, while no TSC patient harboring rhinencephalon hamartomas had concomitant forebrain sulcation abnormalities. None of the patients showed overt olfactory deficits or hypogonadism, though young age and poor compliance hampered a proper evaluation in most cases. TSC patients with rhinencephalon changes had more cortical tubers (47 ± 29.1 vs 26.2 ± 19.6; p = 0.006) but did not differ for clinical severity (p = 0.45) compared to the other patients of the sample.
Conclusions
Olfactory bulb and/or forebrain changes are not rare among TSC subjects. Future studies investigating clinical consequences in older subjects (anosmia, gonadic development etc.) will define whether rhinencephalon changes are simply an imaging feature among the constellation of TSC-related brain changes or a feature to be searched for possible implications in the management of TSC subjects.





Similar content being viewed by others
References
Randle SC (2017) Tuberous sclerosis complex: a review. Pediatr Ann 46(4):e166–e171
Curatolo P, Moavero R, de Vries PJ (2015) Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol 14:733–745
Northrup H, Krueger DA, on behalf of the International Tuberous Sclerosis Complex Consensus Group: Tuberous Sclerosis Complex Diagnostic Criteria Update (2013) Recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 49:243–254
Kingswood C, Bolton P, Crawford P, Harland C, Johnson SR, Sampson JR, Shepherd C, Spink J, Demuth D, Lucchese L, Nasuti P, Gray E, Pinnegar A, Magestro M (2016) The clinical profile of tuberous sclerosis complex (TSC) in the United Kingdom: a retrospective cohort study in the Clinical Practice Research Datalink (CPRD). Eur J Paediatr Neurol 20(2):296–308
Boer K, Troost D, Jansen F, Nellist M, van den Ouweland AM, Geurts JJ, Spliet WG, Crino P, Aronica E (2008) Clinicopathological and immunohistochemical findings in an autopsy case of tuberous sclerosis complex. Neuropathology 28(6):577–590
Boronat S, Shaaya EA, Auladell M, Thiele EA, Caruso P (2014) Intracranial arteriopathy in tuberous sclerosis complex. J Child Neurol 29:912–919
Tortori-Donati P, Rossi A (2005) Pediatric neuroradiology brain. Springer-Verlag, Berlin, pp 785–800
Sarnat HB, Flores-Sarnat L (2017) Olfactory development, part 2: neuroanatomic maturation and dysgeneses. J Child Neurol 32(6):579–593
Manara R, Salvalaggio A, Favaro A, Palumbo V, Citton V, Elefante A, Brunetti A, Di Salle F, Bonanni G, Sinisi AA (2014) Kallmann syndrome neuroradiological study group. Brain changes in Kallmann syndrome. AJNR Am J Neuroradiol 35(9):1700–1706
Quinton R, Duke VM, de Zoysa PA, Platts AD, Valentine A, Kendall B, Pickman S, Kirk JM, Besser GM, Jacobs HS, Bouloux PM (1996) The neuroradiology of Kallmann’s syndrome: a genotypic and phenotypic analysis. J Clin Endocrinol Metab 81(8):3010–3017
de León GA, Zaeri N, Foley CM (1988) Olfactory hamartomas in tuberous sclerosis. J Neurol Sci 87(2–3):187–194
Feliciano DM, Quon JL, Su T, Taylor MM, Bordey A (2012) Postnatal neurogenesis generates heterotopias, olfactory micronodules and cortical infiltration following single-cell Tsc1 deletion. Hum Mol Genet 21(4):799–810
Wong AM, Wang HS, Schwartz ES, Toh CH, Zimmerman RA, Liu PL, Wu YM, Ng SH, Wang JJ (2013) Cerebral diffusion tensor MR tractography in tuberous sclerosis complex: correlation with neurologic severity and tract-based spatial statistical analysis. AJNR Am J Neuroradiol 34:1829–1835
Ottaviano G, Cantone E, D'Errico A, Salvalaggio A, Citton V, Scarpa B, Favaro A, Sinisi AA, Liuzzi R, Bonanni G, Di Salle F, Elefante A, Manara R, Staffieri A, Martini A, Brunetti A (2015) Sniffin’ Sticks and olfactory system imaging in patients with Kallmann syndrome. Int Forum Allergy Rhinol 5(9):855–861
Cabrera SM, Rogol AD (2013) Testosterone exposure in childhood: discerning pathology from physiology. Expert Opin Drug Saf 12(3):375–388
Manara R, Bugin S, Pelizza MF, Sartori S, Nosadini M, Labriola F, Canevini P, Vignoli A, Toldo I (2018) Genetic and imaging features of cerebellar abnormalities in tuberous sclerosis complex: more insights into their pathogenesis. Dev Med Child Neurol 60(7):724-725
Boronat S, Thiele EA, Caruso P (2017) Cerebellar lesions are associated with TSC2 mutations in tuberous sclerosis complex: a retrospective record review study. Dev Med Child Neurol 59(10):1071–1076
Au KS, Williams AT, Roach ES, Batchelor L, Sparagana SP, Delgado MR, Wheless JW, Baumgartner JE, Roa BB, Wilson CM, Smith-Knuppel TK, Cheung MY, Whittemore VH, King TM, Northrup H (2007) Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet Med 9:88–100
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
For this type of retrospective study formal consent is not required.
Electronic supplementary material
e-Fig. 1
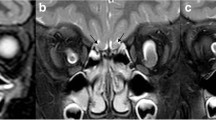
MRI of normal olfactory bulbs and sulci in tuberous sclerosis complex patients; magnifications of rhinencephalon and forebrain details are on the right. A) coronal T2-weighted image disclosing the oval shape of the anterior rhinencephalon (short arrows) within the olfactory grooves; the olfactory sulci indent the inferior surface of the frontal lobes about one centimeter from the midline (arrowheads); several cortical tubers are recognizable, especially in the right frontal lobe B) axial image at the level of the upper portion of the orbits showing the olfactory sulci (arrowheads) separating the giri recti from the remaining orbitofrontal cortex; the crista galli (*) is also recognizable; C) Parasagittal T2-weighted image disclosing the cigar-like olfactory bulb (short arrows). Subependymal nodules and giant astrocytoma are evident close to Monro’s foramina. (PNG 1341 kb)
Rights and permissions
About this article
Cite this article
Manara, R., Brotto, D., Bugin, S. et al. Rhinencephalon changes in tuberous sclerosis complex. Neuroradiology 60, 813–820 (2018). https://doi.org/10.1007/s00234-018-2045-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-018-2045-x