Abstract
Purpose
Altered brain functional connectivity has been reported in patients with amblyopia by recent neuroimaging studies. However, relatively little is known about the alterations in interhemispheric functional connectivity in amblyopia. The present study aimed to investigate the functional connectivity patterns between homotopic regions across hemispheres in patients with anisometropic and strabismic amblyopia under resting state.
Methods
Nineteen monocular anisometropic amblyopia (AA), 18 strabismic amblyopia (SA), and 20 normal-sight controls (NC) were enrolled in this study. After a comprehensive ophthalmologic examination, resting-state fMRI scanning was performed in all participants. The pattern of the interhemispheric functional connectivity was measured with the voxel-mirrored homotopic connectivity (VMHC) approach. VMHC values differences within and between three groups were compared, and correlations between VMHC values and each the clinical variable were also analyzed.
Results
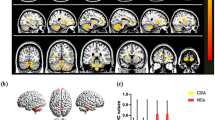
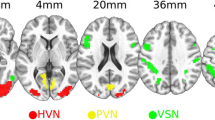
Altered VMHC was observed in AA and SA patients in lingual gyrus and fusiform gyrus compared with NC subjects. The altered VMHC of lingual gyrus showed a pattern of AA > SA > NC, while the altered VMHC of fusiform gyrus showed a pattern of AA > NC > SA. Moreover, the VMHC values of lingual gyrus were positively correlated with the stereoacuity both in AA and SA patients, and the VMHC values of fusiform gyrus were positively correlated with the amount of anisometropia just in AA patients.
Conclusion
These findings suggest that interhemispheric functional coordination between several homotopic visual-related brain regions is impaired both in AA and SA patients under resting state and revealed the similarities and differences in interhemispheric functional connectivity between the anisometropic and strabismic amblyopia.



Similar content being viewed by others
Abbreviations
- AA:
-
Anisometropic amblyopia
- SA:
-
Strabismic amblyopia
- NC:
-
Normal-sight controls
- rs-fMRI:
-
Resting-state functional magnetic resonance
- RSFC:
-
Resting-state functional connectivity
- VMHC:
-
Voxel-mirrored homotopic connectivity
- cVA:
-
Corrected visual acuity
- GRF:
-
Gaussian random field
- FFA:
-
Fusiform face area
References
Holmes JM, Clarke MP (2006) Amblyopia. Lancet 367(9519):1343–1351. doi:10.1016/S0140-6736(06)68581-4
Levi DM (2013) Linking assumptions in amblyopia. Vis Neurosci 30(5–6):277–287. doi:10.1017/S0952523813000023
Hamm LM, Black J, Dai S, Thompson B (2014) Global processing in amblyopia: a review. Front Psychol 5:583. doi:10.3389/fpsyg.2014.00583
Noorden GK (1977) Mechanisms of amblyopia. Advances in ophthalmology = Fortschritte der Augenheilkunde = Progres en ophtalmologie 34:93–115
Joly O, Franko E (2014) Neuroimaging of amblyopia and binocular vision: a review. Front Integr Neurosci 8:62. doi:10.3389/fnint.2014.00062
Wang T, Li Q, Guo M, Peng Y, Li Q, Qin W, Yu C (2014) Abnormal functional connectivity density in children with anisometropic amblyopia at resting-state. Brain Res 1563:41–51. doi:10.1016/j.brainres.2014.03.015
Ding K, Liu Y, Yan X, Lin X, Jiang T (2013) Altered functional connectivity of the primary visual cortex in subjects with amblyopia. Neural plasticity 2013:612086. doi:10.1155/2013/612086
Keenan PA, Whitman RD, Pepe J (1989) Hemispheric asymmetry in the processing of high and low spatial frequencies: a facial recognition task. Brain Cogn 11(2):229–237
Rossion B, Dricot L, Devolder A, Bodart JM, Crommelinck M, De Gelder B, Zoontjes R (2000) Hemispheric asymmetries for whole-based and part-based face processing in the human fusiform gyrus. J Cogn Neurosci 12(5):793–802
Sergent J, Bindra D (1981) Differential hemispheric processing of faces: methodological considerations and reinterpretation. Psychol Bull 89(3):541–554
Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, Grzadzinski R, Evans AC, Zang YF, Castellanos FX, Milham MP (2010) Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. The Journal of neuroscience : the official journal of the Society for Neuroscience 30(45):15034–15043. doi:10.1523/JNEUROSCI.2612-10.2010
Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8(9):700–711. doi:10.1038/nrn2201
Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 34(4):537–541
Stark DE, Margulies DS, Shehzad ZE, Reiss P, Kelly AM, Uddin LQ, Gee DG, Roy AK, Banich MT, Castellanos FX, Milham MP (2008) Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. The Journal of neuroscience : the official journal of the Society for Neuroscience 28(51):13754–13764. doi:10.1523/JNEUROSCI.4544-08.2008
Hoptman MJ, Zuo XN, D’Angelo D, Mauro CJ, Butler PD, Milham MP, Javitt DC (2012) Decreased interhemispheric coordination in schizophrenia: a resting state fMRI study. Schizophr Res 141(1):1–7. doi:10.1016/j.schres.2012.07.027
Wang L, Li K, Zhang QE, Zeng YW, Jin Z, Dai WJ, Su YA, Wang G, Tan YL, Yu X, Si TM (2013) Interhemispheric functional connectivity and its relationships with clinical characteristics in major depressive disorder: a resting state fMRI study. PLoS One 8(3):e60191. doi:10.1371/journal.pone.0060191
Hu X, Zhang J, Jiang X, Zhou C, Wei L, Yin X, Wu Y, Li J, Zhang Y, Wang J (2015) Decreased interhemispheric functional connectivity in subtypes of Parkinson’s disease. J Neurol 262(3):760–767. doi:10.1007/s00415-014-7627-x
Society CO (2011) Expert consensus on amblyopia diagnosis (2011). Chinese Journal of Ophthalmology 47(8):768
Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF (2011) REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 6(9):e25031. doi:10.1371/journal.pone.0025031
Wallace DK, Lazar EL, Melia M, Birch EE, Holmes JM, Hopkins KB, Kraker RT, Kulp MT, Pang Y, Repka MX, Tamkins SM, Weise KK, Pediatric Eye Disease Investigator G (2011) Stereoacuity in children with anisometropic amblyopia. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus 15(5):455–461. doi:10.1016/j.jaapos.2011.06.007
Yan CG, Wang XD, Zuo XN, Zang YF (2016) DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 14(3):339–351. doi:10.1007/s12021-016-9299-4
Boynton GM, Hegde J (2004) Visual cortex: the continuing puzzle of area V2. Current biology : CB 14(13):R523–R524. doi:10.1016/j.cub.2004.06.044
Salvador R, Martinez A, Pomarol-Clotet E, Gomar J, Vila F, Sarro S, Capdevila A, Bullmore E (2008) A simple view of the brain through a frequency-specific functional connectivity measure. NeuroImage 39(1):279–289. doi:10.1016/j.neuroimage.2007.08.018
von der Heydt R, Zhou H, Friedman HS (2000) Representation of stereoscopic edges in monkey visual cortex. Vis Res 40(15):1955–1967
Qiu FT, von der Heydt R (2005) Figure and ground in the visual cortex: v2 combines stereoscopic cues with gestalt rules. Neuron 47(1):155–166. doi:10.1016/j.neuron.2005.05.028
Willmore BD, Prenger RJ, Gallant JL (2010) Neural representation of natural images in visual area V2. The Journal of neuroscience : the official journal of the Society for Neuroscience 30(6):2102–2114. doi:10.1523/JNEUROSCI.4099-09.2010
Levi DM, Knill DC, Bavelier D (2015) Stereopsis and amblyopia: a mini-review. Vis Res 114:17–30. doi:10.1016/j.visres.2015.01.002
McKee SP, Levi DM, Movshon JA (2003) The pattern of visual deficits in amblyopia. J Vis 3(5):380–405. doi:10.1167/3.5.5
Li G, Yao Z, Wang Z, Yuan N, Talebi V, Tan J, Wang Y, Zhou Y, Baker CL Jr (2014) Form-cue invariant second-order neuronal responses to contrast modulation in primate area V2. The Journal of neuroscience : the official journal of the Society for Neuroscience 34(36):12081–12092. doi:10.1523/JNEUROSCI.0211-14.2014
Henriksson L, Nurminen L, Hyvarinen A, Vanni S (2008) Spatial frequency tuning in human retinotopic visual areas. J Vis 8(10):5.1–513. doi:10.1167/8.10.5
Kanwisher N, McDermott J, Chun MM (1997) The fusiform face area: a module in human extrastriate cortex specialized for face perception. The Journal of neuroscience : the official journal of the Society for Neuroscience 17(11):4302–4311
Le Grand R, Mondloch CJ, Maurer D, Brent HP (2001) Neuroperception. Early visual experience and face processing. Nature 410(6831):890. doi:10.1038/35073749
Lerner Y, Pianka P, Azmon B, Leiba H, Stolovitch C, Loewenstein A, Harel M, Hendler T, Malach R (2003) Area-specific amblyopic effects in human occipitotemporal object representations. Neuron 40(5):1023–1029
Banko EM, Kortvelyes J, Nemeth J, Weiss B, Vidnyanszky Z (2013a) Amblyopic deficits in the timing and strength of visual cortical responses to faces. Cortex; a journal devoted to the study of the nervous system and behavior 49(4):1013–1024. doi:10.1016/j.cortex.2012.03.021
Cattaneo Z, Vecchi T, Monegato M, Pece A, Merabet LB, Carbon CC (2013) Strabismic amblyopia affects relational but not featural and gestalt processing of faces. Vis Res 80:19–30. doi:10.1016/j.visres.2013.01.007
Banko EM, Kortvelyes J, Weiss B, Vidnyanszky Z (2013b) How the visual cortex handles stimulus noise: insights from amblyopia. PLoS One 8(6):e66583. doi:10.1371/journal.pone.0066583
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the Southwest Hospital Ethics Committee and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Liang, M., Xie, B., Yang, H. et al. Altered interhemispheric functional connectivity in patients with anisometropic and strabismic amblyopia: a resting-state fMRI study. Neuroradiology 59, 517–524 (2017). https://doi.org/10.1007/s00234-017-1824-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-017-1824-0