Abstract
Introduction
Complaint about attention disorders is common among breast cancer patients who have undergone chemotherapy, which may be associated with the default mode network (DMN). To validate this hypothesis, we investigated the DMN functional connectivity (FC) change and its relationship with the attention function in breast cancer patients (BC) using resting-state functional magnetic resonance imaging (rs-fMRI).
Methods
Twenty-two BC treated with chemotherapy and 22 healthy controls (HC) were recruited into this study. The FC between the DMN’s hubs and regions of the dorsal medial prefrontal cortex (dMPFC) and medial temporal lobe (MTL) subsystems was respectively calculated for each participant.
Results
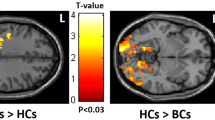
The statistical result showed significantly lower connectivity in dMPFC and MTL subsystems in the BC group. In addition, the partial correlation analysis result indicated that the low connectivity of some brain regions in MTL subsystem was correlated with attention dysfunction following BC chemotherapy.
Conclusion
These results suggest that the functional disconnection in MTL subsystem of the DMN may have association with attention function of BC after chemotherapy.



Similar content being viewed by others
References
Jim HS, Phillips KM, Chait S, Faul LA, Popa MA, Lee YH, Hussin MG, Jacobsen PB, Small BJ (2012) Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol 30(29):3578–3587. doi:10.1200/JCO.2011.39.5640
Kaemingk K, Lundy S, Patton T, Remninger S, Kervick R (2006) Attention is frequently weaker than would be expected based on intelligence in breast cancer survivors treated with chemotherapy. In: Psycho-oncology. vol 1. John Wiley & Sons Ltd The Atrium, Southern Gate, Chichester PO19 8SQ, W Sussex, England, pp S3–S4
Chen X, Li J, Ren J, Hu X, Zhu C, Tian Y, Hu P, Ma H, Yu F, Wang K (2014) Selective impairment of attention networks in breast cancer patients receiving chemotherapy treatment. Psychooncology 23(10):1165–1171. doi:10.1002/pon.3539
Bender CM, Sereika SM, Berga SL, Vogel VG, Brufsky AM, Paraska KK, Ryan CM (2006) Cognitive impairment associated with adjuvant therapy in breast cancer. Psychooncology 15(5):422–430. doi:10.1002/pon.964
Deprez S, Amant F, Smeets A, Peeters R, Leemans A, Van Hecke W, Verhoeven JS, Christiaens MR, Vandenberghe J, Vandenbulcke M, Sunaert S (2012) Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol 30(3):274–281. doi:10.1200/JCO.2011.36.8571
Chan RC, Shum D, Toulopoulou T, Chen EY (2008) Assessment of executive functions: review of instruments and identification of critical issues. Arch Clin Neuropsychol : Off J Natl Acad Neuropsychol 23(2):201–216. doi:10.1016/j.acn.2007.08.010
Damoiseaux JS, Prater KE, Miller BL, Greicius MD (2012) Functional connectivity tracks clinical deterioration in Alzheimer’s disease. Neurobiol Aging 33(4):828. doi:10.1016/j.neurobiolaging.2011.06.024, e819-830
Friston KJ, Frith CD (1995) Schizophrenia: a disconnection syndrome? Clin Neurosci 3(2):89–97
Binnewijzend MA, Schoonheim MM, Sanz-Arigita E, Wink AM, van der Flier WM, Tolboom N, Adriaanse SM, Damoiseaux JS, Scheltens P, van Berckel BN, Barkhof F (2012) Resting-state fMRI changes in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 33(9):2018–2028. doi:10.1016/j.neurobiolaging.2011.07.003
Kesler SR, Wefel JS, Hosseini SM, Cheung M, Watson CL, Hoeft F (2013) Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proc Natl Acad Sci U S A 110(28):11600–11605. doi:10.1073/pnas.1214551110
Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP (2009) Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp 30(2):625–637. doi:10.1002/hbm.20531
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci U S A 98(2):676–682. doi:10.1073/pnas.98.2.676
Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH (2012) The role of default network deactivation in cognition and disease. Trends Cogn Sci 16(12):584–592. doi:10.1016/j.tics.2012.10.008
Andrews-Hanna JR (2009) The brain’s default network: anatomy, function, and consequence of disruption. Harvard University
Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, Akechi T, Wada N, Imoto S, Murakami K, Uchitomi Y (2007) Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer 109(1):146–156. doi:10.1002/cncr.22368
McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ (2010) Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat 123(3):819–828. doi:10.1007/s10549-010-1088-4
Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, Dhabhar FS (2013) Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun 30(Suppl):S109–S116. doi:10.1016/j.bbi.2012.05.017
Uddin LQ, Kelly AC, Biswal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler LA, Castellanos FX (2008) Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods 169(1):249–254
Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ (2009) Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev 33(3):279–296. doi:10.1016/j.neubiorev.2008.09.002
Fransson P (2006) How default is the default mode of brain function?: further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia 44(14):2836–2845
Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB (2009) A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res 1273:114–128
Cabeza R, Nyberg L (2000) Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12(1):1–47
Nugent AC, Milham MP, Bain EE, Mah L, Cannon DM, Marrett S, Zarate CA, Pine DS, Price JL, Drevets WC (2006) Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. NeuroImage 30(2):485–497. doi:10.1016/j.neuroimage.2005.09.029
Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS (2005) Volume of the cingulate and outcome in schizophrenia. Schizophr Res 72(2–3):91–108. doi:10.1016/j.schres.2004.02.011
Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, Theberge J, Schaefer B, Williamson PC (2009) Retrosplenial cortex connectivity in schizophrenia. Psychiatry Res 174(1):17–23. doi:10.1016/j.pscychresns.2009.03.010
Dudukovic NM, Preston AR, Archie JJ, Glover GH, Wagner AD (2011) High-resolution fMRI reveals match enhancement and attentional modulation in the human medial temporal lobe. J Cogn Neurosci 23(3):670–682. doi:10.1162/jocn.2010.21509
So WK, Dodgson J, Tai JW (2003) Fatigue and quality of life among Chinese patients with hematologic malignancy after bone marrow transplantation. Cancer Nurs 26(3):211–219
Lezak MD (1984) Neuropsychological assessment in behavioral toxicology—developing techniques and interpretative issues. Scand J Work Environ Health :25–29
Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18(6):643
MacLeod CM (1991) Half a century of research on the Stroop effect: an integrative review. Psychol Bull 109(2):163–203
MacLeod CM, MacDonald PA (2000) Interdimensional interference in the Stroop effect: uncovering the cognitive and neural anatomy of attention. Trends Cogn Sci 4(10):383–391
Kane MJ, Conway AR, Hambrick DZ, Engle RW (2007) Variation in working memory capacity as variation in executive attention and control. Var Work Mem 1:21–48
Lamers MJ, Roelofs A, Rabeling-Keus IM (2010) Selective attention and response set in the Stroop task. Mem Cogn 38(7):893–904. doi:10.3758/MC.38.7.893
Zarghi A, Zali A, Tehranidost M, Ashrafi F, Zarindast M, Moazezi M, Khodadadi S (2012) Assessment of selective attention with cscwt (computerized stroop color-word test) among children and adults. US-China Educ Rev A 1:121–127
Barkley RA (1997) Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121(1):65
Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010) Functional-anatomic fractionation of the brain’s default network. Neuron 65(4):550–562
Naghavi HR, Nyberg L (2005) Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Conscious Cogn 14(2):390–425. doi:10.1016/j.concog.2004.10.003
Coull JT, Frith CD, Frackowiak RS, Grasby PM (1996) A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia 34(11):1085–1095
Dalley JW, Cardinal RN, Robbins TW (2004) Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28(7):771–784. doi:10.1016/j.neubiorev.2004.09.006
Arnsten AF, Li BM (2005) Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry 57(11):1377–1384. doi:10.1016/j.biopsych.2004.08.019
Sara SJ (2009) The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 10(3):211–223. doi:10.1038/nrn2573
Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001) Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A 98(7):4259–4264. doi:10.1073/pnas.071043098
Elliott R, Dolan RJ, Frith CD (2000) Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex 10(3):308–317
Vann SD, Aggleton JP, Maguire EA (2009) What does the retrosplenial cortex do? Nat Rev Neurosci 10(11):792–802
Sugar J, Witter MP, van Strien NM, Cappaert NL (2011) The retrosplenial cortex: intrinsic connectivity and connections with the (para)hippocampal region in the rat. An interactive connectome. Front Neuroinformatics 5:7. doi:10.3389/fninf.2011.00007
Vann SD, Wilton LK, Muir JL, Aggleton JP (2003) Testing the importance of the caudal retrosplenial cortex for spatial memory in rats. Behav Brain Res 140(1):107–118
Gazzaley A, Nobre AC (2012) Top-down modulation: bridging selective attention and working memory. Trends Cogn Sci 16(2):129–135. doi:10.1016/j.tics.2011.11.014
Critchleey M (1953) Parietal lobes. Giornale di Psichiatria e di Neuropatologia 81(4):872–873
Perry RJ, Hodges JR (1999) Attention and executive deficits in Alzheimer’s disease. A critical review. Brain 122(Pt 3):383–404
Caspers S, Schleicher A, Bacha-Trams M, Palomero-Gallagher N, Amunts K, Zilles K (2013) Organization of the human inferior parietal lobule based on receptor architectonics. Cereb Cortex 23(3):615–628. doi:10.1093/cercor/bhs048
Visintin E, De Panfilis C, Antonucci C, Capecci C, Marchesi C, Sambataro F (2015) Parsing the intrinsic networks underlying attention: a resting state study. Behav Brain Res 278:315–322
Xi Q, Zhao XH, Wang PJ, Guo QH, He Y (2013) Abnormal intrinsic brain activity in amnestic mild cognitive impairment revealed by amplitude of low-frequency fluctuation: a resting-state functional magnetic resonance imaging study. Chin Med J 126(15):2912–2917
Tuxen MK, Hansen SW (1994) Neurotoxicity secondary to antineoplastic drugs. Cancer Treat Rev 20(2):191–214
Joseph JA, Denisova N, Fisher D, Shukitt-Hale B, Bickford P, Prior R, Cao G (1998) Age-related neurodegeneration and oxidative stress: putative nutritional intervention. Neurol Clin 16(3):747–755
Ahles TA (2004) Do systemic cancer treatments affect cognitive function? Lancet Oncol 5(5):270–271. doi:10.1016/s1470-2045(04)01463-9
Barton D, Loprinzi C (2002) Novel approaches to preventing chemotherapy-induced cognitive dysfunction in breast cancer: the art of the possible. Clin Breast Cancer 3:S121–S127
Costa SD, von Minckwitz G, Raab G, Blohmer JU, Dresel V, Eidtmann H, Hilfrich J, Jackisch C, Merkle E, Gademann G, Kaufmann M (1999) The role of docetaxel (Taxotere) in neoadjuvant chemotherapy of breast cancer. Semin Oncol 26(3 Suppl 9):24–31
Saykin A, Ahles T, McDonald B (2003) Mechanisms of chemotherapy-induced cognitive disorders: neuropsychological, pathophysiological, and neuroimaging perspectives. Semin Clin Neuropsychiatry 4:201–216
Langer N, von Bastian CC, Wirz H, Oberauer K, Jancke L (2013) The effects of working memory training on functional brain network efficiency. Cortex; J Devoted Study Nervous Syst Behav 49(9):2424–2438. doi:10.1016/j.cortex.2013.01.008
Acknowledgments
This work was supported by National Science Foundation of China (Grant numbers: 81371537, 91432301), Major State Basic Research Development Program of China (973 Program) (Grant number: 2013CB733803) and the Fundamental Research Funds for the Central Universities of China (WK2070000033).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
We declare that all human and animal studies have been approved by the research ethics committee of the First Affiliated Hospital of Anhui Medical University and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Additional information
HM and XC contributed equally to this work.
Rights and permissions
About this article
Cite this article
Miao, H., Chen, X., Yan, Y. et al. Functional connectivity change of brain default mode network in breast cancer patients after chemotherapy. Neuroradiology 58, 921–928 (2016). https://doi.org/10.1007/s00234-016-1708-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-016-1708-8