Abstract
Introduction
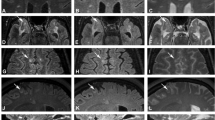
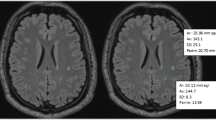
Regional brain volume estimation in multiple sclerosis (MS) patients is prone to error due to white matter lesions being erroneously segmented as grey matter. The Lesion Segmentation Toolbox (LST) is an automatic tool that estimates a lesion mask based on 3D T2-FLAIR images and then uses this mask to fill the structural MRI image. The goal of this study was (1) to test the LST for estimating white matter lesion volume in a cohort of MS patients using 2D T2-FLAIR images, and (2) to evaluate the performance of the optimized LST on image segmentation and the impact on the calculated grey matter fraction (GMF).
Methods
The study included 110 patients with a clinically isolated syndrome and 42 with a relapsing-remitting MS scanned on a 3.0-T MRI system. In a subset of consecutively selected patients, the lesion mask was semi-manually delineated over T2-FLAIR images. After establishing the optimized LST parameters, the corresponding regional fractions were calculated for the original, filled, and masked images.
Results
A high agreement (intraclass correlation coefficient (ICC) = 0.955) was found between the (optimized) LST and the semi-manual lesion volume estimations. The GMF was significantly smaller when lesions were masked (mean difference −0.603, p < 0.001) or when the LST filling technique was used (mean difference −0.598, p < 0.001), compared to the GMF obtained from the original image.
Conclusion
LST lesion volume calculation seems reliable. GMFs are significantly reduced when a method to correct the contribution of MS lesions is used, and it may have an impact in assessing GMF differences between clinical cohorts.






Similar content being viewed by others
Abbreviations
- ANOVA:
-
One-way analysis of variance
- CIS:
-
Clinically isolated syndromes
- DC:
-
Dice coefficient
- EDSS:
-
Expanded disability status scale
- GM:
-
Grey matter
- GMF:
-
Grey matter fraction
- GT:
-
Ground truth
- ICC:
-
Intraclass correlation coefficient
- LST:
-
Lesion Segmentation Toolbox
- LV:
-
Lesion volume
- MNI:
-
Montreal Neurological Institute
- MRI:
-
Magnetic resonance imaging
- MS:
-
Multiple sclerosis
- RRMS:
-
Relapsing-remitting multiple sclerosis
- SPM:
-
Statistical Parametric Mapping
- VBM:
-
Voxel-based morphometry
- WM:
-
White matter
- WMF:
-
White matter fraction
References
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372:1502–17
Lassmann H (2008) The pathologic substrate of magnetic resonance alterations in multiple sclerosis. Neuroimaging Clin N Am 18(4):563–76
Filippi M, Rocca MA, Barkhof F, Brück W, Chen JT, Comi G et al (2012) Attendees of the correlation between pathological MRI findings in MS workshop. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol 11:349–60
Bermel RA, Bakshi R (2006) The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol 5(2):158–70
Filippi M (2015) MRI measures of neurodegeneration in multiple sclerosis: implications for disability, disease monitoring, and treatment. J Neurol 262(1):1–6
De Stefano N, Airas L, Grigoriadis N, Mattle HP, O'Riordan J, Oreja-Guevara C et al (2014) Clinical relevance of brain volume measures in multiple sclerosis. CNS Drugs 28:147–56
Derakhshan M, Caramanos Z, Giacomini PS, Narayanan S, Maranzano J, Francis SJ, Arnold DL, Collins DL (2010) Evaluation of automated techniques for the quantification of grey matter atrophy in patients with multiple sclerosis. Neuroimage 52:1261–7
Chard DT, Jackson JS, Miller DH, Wheeler-Kingshott CA (2010) Reducing the impact of white matter lesions on automated measures of brain gray and white matter volumes. J Magn Reson Imaging 32:223–8
Schmidt P, Gaser C, Arsic M, Buck D, Förschler A, Berthele A et al (2012) An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage 59:3774–83
Maldjian JA, Whitlow CT, Saha BN, Kota G, Vandergriff C, Davenport EM et al (2013) Automated white matter total lesion volume segmentation in diabetes. AJNR Am J Neuroradiol 34:2265–70
Ashburner J, Friston KJ (2000) Voxel-based morphometry—the methods. Neuroimage 11:805–21
Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38(1):95–113
Gelineau-Morel R, Tomassini V, Jenkinson M, Johansen-Berg H, Matthews PM, Palace J (2012) The effect of hypointense white matter lesions on automated gray matter segmentation in multiple sclerosis. Hum Brain Mapp 33:2802–14
Acknowledgments
We thank the Red Española de Esclerosis Múltiple (REEM) (RD07/0060; RD12/0032), which is sponsored by the Fondo de Investigación Sanitaria (FIS), Instituto de Salud Carlos III, Ministry of Economy and Competitiveness in Spain, and the Ajuts per donar Suport als Grups de Recerca de Catalunya (2009 SGR 0793), which is sponsored by the Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR) of the Catalonian regional government (Generalitat de Catalunya) in Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We declare that this manuscript does not contain clinical studies or patient data.
Conflict of interest
DP has received speaking honoraria from Novartis and Genzyme. JSG has received compensation for consulting services and speaking honoraria from Merck-Serono, Biogen, Teva, Genzyme, Almirall, Novartis and Excemed. CA has received speaking honoraria from Novartis and Genzyme. MT has received compensation for consulting services and speaking honoraria from Almirall, Bayer, Biogen, Genzyme, Merck, Novartis, Roche, Sanofi-Aventis and Teva, and has been on advisory boards of Biogen, Genzyme and Teva; grants paid to the institution by Biogen and Novartis. XM has received speaking honoraria and travel expenses for scientific meetings, has been a steering committee member of clinical trials, or participated in advisory boards of clinical trials in the past with Actelion, Almirall, Bayer-Schering, Biogen, F. Hoffman - La Roche, Merck-Serono, Genentech, Genzyme, Novartis, Receptos, Sanofi and Teva; grants paid to the institution from Octopharma and Thropos. AR serves on scientific advisory boards and/or has received speaker honoraria from Biogen, Novartis, Genzyme, OLEA Medical, Bayer, Bracco, Merck-Serono, Teva and Stendhal.
Rights and permissions
About this article
Cite this article
Pareto, D., Sastre-Garriga, J., Aymerich, F.X. et al. Lesion filling effect in regional brain volume estimations: a study in multiple sclerosis patients with low lesion load. Neuroradiology 58, 467–474 (2016). https://doi.org/10.1007/s00234-016-1654-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-016-1654-5