Abstract
Introduction
Neuromelanin-sensitive MR imaging (MRI) can visualize neuromelanin-containing neurons in the substantia nigra pars compacta (SNc), and its utility has been reported in the evaluation of parkinsonism. Conversely, dopamine transporter imaging by 123I-N-v-fluoropropyl-2b-carbomethoxy-3b-(4-iodophenyl)nortropane (FP-CIT) SPECT (DaTSCAN) is now an established method for evaluating parkinsonism, detecting presynaptic dopamine neuronal dysfunction. Both methods can assist differentiating neurodegenerative and other forms of parkinsonism. However, to our knowledge, there have been no studies concerning a correlation between the two methods. The aim of this study was to assess the utility of neuromelanin-sensitive MRI for diagnosing parkinsonism by examining a correlation with DaTSCAN.
Methods
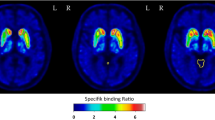
Twenty-three patients with parkinsonism who underwent both neuromelanin-sensitive MRI and DaTSCAN were included. We measured the neuromelanin-positive SNc region volume by manually contouring the high signal intensity region of the SNc on neuromelanin-sensitive MRI and measured the specific binding ratio (SBR) on DaTSCAN. The asymmetry index of neuromelanin-positive SNc volume and the asymmetry index of SBR were also calculated.
Results
The volume of the neuromelanin-positive SNc region showed significant correlation with specific binding ratio (SBR) (right P < .001, ρ = 0.78, left P < .001, ρ = 0.86). The asymmetry index of neuromelanin-positive SNc volume also showed significant correlations with the asymmetry index of SBR (P < .001, ρ = 0.73).
Conclusions
Decrease of the high signal intensity region of the SNc on neuromelanin-sensitive MRI would indicate damage to the nigrostriatal dopaminergic function as well as loss of dopaminergic neurons. We conclude that neuromelanin-sensitive MRI is a useful diagnostic biomarker for parkinsonism.



Similar content being viewed by others
References
Fearnley JM, Lees AJ (1991) Aging and Parkinson’s disease: substantia nigra regional selectivity. Brain 114:2283–2301
Ozawa T, Paviour D, Quinn NP, Josephs KA, Sangha H, Kilford L, Healy DG, Wood NW, Lees AJ, Holton JL, Revesz T (2004) The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain 127:2657–2671
Morris H, Wood N, Lees A (1999) Progressive supranuclear palsy (Steele-Richardson-Olszewski disease). Postgrad Med J 75:579–584
Josephs KA, Tang-Wai DF, Edland SD, Knopman DS, Dickson DW, Parisi JE, Petersen RC, Jack CR Jr, Boeve BF (2004) Correlation between antemortem magnetic resonance imaging findings and pathologically confirmed corticobasal degeneration. Arch Neurol 61:1881–1884
Graham DG (1979) On the origin and significance of neuromelanin. Arch Pathol Lab Med 103:359–362
Bazelon M, Fenichel GM, Randall J (1967) Studies on neuromelanin: I. A melanin system in the human adult brainstem. Neurology 17:512–519
Stepień K, Wilczok A, Zajdel A, Dzierzega-Lecznar A, Wilczok T (2000) Peroxynitrite mediated linoleic acid oxidation and tyrosine nitration in the presence of synthetic neuromelanins. Acta Biochim Pol 47:931–940
Kooncumchoo P, Sharma S, Porter J, Govitrapong P, Ebadi M (2006) Coenzyme Q(10) provides neuroprotection in iron-induced apoptosis in dopaminergic neurons. J Mol Neurosci 28:125–141
Sharma S, Moon CS, Khogali A, Haidous A, Chabenne A, Ojo C, Jelebinkov M, Kurdi Y, Ebadi M (2013) Biomarkers in Parkinson's disease (recent update). Neurochem Int 63:201–229. doi:10.1016/j.neuint.2013.06.005
Sasaki M, Shibata E, Tohyama K, Takahashi J, Otsuka K, Tsuchiya K, Takahashi S, Ehara S, Terayama Y, Sakai A (2006) Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson’s disease. NeuroReport 17:1215–1218
Mann DM, Yates PO (1983) Possible role of neuromelanin in the pathogenesis of Parkinson’s disease. Mech Ageing Dev 21:193–203
Schwarz ST, Rittman T, Gontu V, Morgan PS, Bajaj N, Auer DP (2011) T1-weighted MRI shows stage-dependent substantia nigra signal loss in Parkinson’s disease. Mov Disord 26:1633–1638. doi:10.1002/mds.23722
Kashihara K, Shinya T, Higaki F (2011) Neuromelanin magnetic resonance imaging of nigral volume loss in patients with Parkinson’s disease. J Clin Neurosci 18:1093–1096. doi:10.1016/j.jocn.2010.08.043
Nikolaus S, Antke C, Kley K, Poeppel TD, Hautzel H, Schmidt D, Müller HW (2007) Investigating the dopaminergic synapse in vivo: I. Molecular imaging studies in humans. Rev Neurosci 18:439–472
Nikolaus S, Antke C, Muller HW (2009) In vivo imaging of synaptic function in the central nervous system: I. Movement disorders and dementia. Behav Brain Res 204:1–31. doi:10.1016/j.bbr.2009.06.008
la Fougère C, Pöpperl G, Levin J, Wängler B, Böning G, Uebleis C, Cumming P, Bartenstein P, Bötzel K, Tatsch K (2010) The value of the dopamine D2/3 receptor ligand 18F-desmethoxyfallypride for the differentiation of idiopathic and nonidiopathic parkinsonian syndromes. J Nucl Med 51:581–587. doi:10.2967/jnumed.109.071811
Kim YJ, Ichise M, Ballinger JR, Vines D, Erami SS, Tatschida T, Lang AE (2002) Combination of dopamine transporter and D2 receptor SPECT in the diagnostic evaluation of PD, MSA, and PSP. Mov Disord 17:303–312
Südmeyer M, Antke C, Zizek T, Beu M, Nikolaus S, Wojtecki L, Schnitzler A, Müller HW (2011) Diagnostic accuracy of combined FP-CIT, IBZM, and MIBG scintigraphy in the differential diagnosis of degenerative parkinsonism: a multidimensional statistical approach. J Nucl Med 52:733–740. doi:10.2967/jnumed.110.086959
Kägi G, Bhatia KP, Tolosa E (2010) The role of DAT-SPECT in movement disorders. J Neurol Neurosurg Psychiatry 81:5–12. doi:10.1136/jnnp.2008.157370
Kashihara K, Shinya T, Higaki F (2011) Reduction of neuromelanin-positive nigral volume in patients with MSA, PSP and CBD. Intern Med 50:1683–1687
Dickson JC, Tossici-Bolt L, Sera T, Erlandsson K, Varrone A, Tatsch K, Hutton BF (2010) The impact of reconstruction method on the quantification of DaTSCAN images. Eur J Nucl Med Mol Imaging 37:23–35. doi:10.1007/s00259-009-1212-z
Enochs WS, Hyslop WB, Bennett HF, Brown RD 3rd, Koenig SH, Swartz HM (1997) Sources of the increased longitudinal relaxation rates observed in melanotic melanoma: an in vitro study of synthetic melanins. Investig Radiol 24:794–804
Enochs WS, Petherick P, Bogdanova A, Mohr U, Weissleder R (1997) Paramagnetic metal scavenging by melanin: MR imaging. Radiology 204:417–423
Ohtsuka C, Sasaki M, Konno K, Kato K, Takahashi J, Yamashita F, Terayama Y (2014) Differentiation of early-stage parkinsonisms using neuromelanin-sensitive magnetic resonance imaging. Parkinsonism Relat Disord 20:755–760. doi:10.1016/j.parkreldis.2014.04.005
Kitao S, Matsusue E, Fujii S, Miyoshi F, Kaminou T, Kato S, Ito H, Ogawa T (2013) Correlation between pathology and neuromelanin MR imaging in Parkinson’s disease and dementia with Lewy bodies. Neuroradiology 55:947–953. doi:10.1007/s00234-013-1199-9
Seibyl JP, Marek KL, Quinlan D, Sheff K, Zoghbi S, Zea-Ponce Y, Baldwin RM, Fussell B, Smith EO, Charney DS, van Dyck C et al (1995) Decreased single photon emission computed tomographic [123I]beta-CIT striatal uptake correlates with symptom severity in Parkinson’s disease. Ann Neurol 38:589–598
Colloby SJ, McParland S, O'Brien JT, Attems J (2012) Neuropathological correlates of dopaminergic imaging in Alzheimer’s disease and Lewy body dementias. Brain 135:2798–2808. doi:10.1093/brain/aws211
Litvan I, MacIntyre A, Goetz CG, Wenning GK, Jellinger K, Verny M, Bartko JJ, Jankovic J, McKee A, Brandel JP, Chaudhuri KR, Lai EC, D'Olhaberriague L, Pearce RK, Agid Y (1998) Accuracy of the clinical diagnoses of Lewy body disease, Parkinson disease, and dementia with Lewy bodies: a clinicopathologic study. Arch Neurol 55:969–978
Tissingh G, Bergmans P, Booij J, Winogrodzka A, van Royen EA, Stoof JC, Wolters EC (1998) Drug-naive patients with Parkinson’s disease in Hoehn and Yahr stages I and II show a bilateral decrease in striatal dopamine transporters as revealed by [123I]beta-CIT SPECT. J Neurol 245:14–20
Rossi C, Frosini D, Volterrani D, De Feo P, Unti E, Nicoletti V, Kiferle L, Bonuccelli U, Ceravolo R (2010) Differences in nigro-striatal impairment in clinical variants of early Parkinson’s disease: evidence from a FP-CIT SPECT study. Eur J Neurol 17:626–630
Contrafatto D, Mostile G, Nicoletti A, Dibilio V, Raciti L, Lanzafame S, Luca A, Distefano A, Zappia M (2012) [(123) I]FP-CIT-SPECT asymmetry index to differentiate Parkinson’s disease from vascular parkinsonism. Acta Neurol Scand 126:12–16. doi:10.1111/j.1600-0404.2011.01583.x
Zhang J, Zhang Y, Wang J, Cai P, Luo C, Qian Z, Dai Y, Feng H (2010) Characterizing iron deposition in Parkinson’s disease using susceptibility-weighted imaging: an in vivo MR study. Brain Res 1330:124–130. doi:10.1016/j.brainres.2010.03.036
Baudrexel S, Nürnberger L, Rüb U, Seifried C, Klein JC, Deller T, Steinmetz H, Deichmann R, Hilker R (2010) Quantitative mapping of T1 and T2* discloses nigral and brainstem pathology in early Parkinson’s disease. Neuroimage 51:512–520. doi:10.1016/j.neuroimage.2010.03.005
Barbosa JH, Santos AC, Tumas V, Liu M, Zheng W, Haacke EM, Salmon CE (2015) Quantifying brain iron deposition in patients with Parkinson’s disease using quantitative susceptibility mapping, R2 and R2*. Magn Reson Imaging 33:559–565. doi:10.1016/j.mri.2015.02.021
Haller S, Badoud S, Nguyen D, Barnaure I, Montandon ML, Lovblad KO, Burkhard PR (2013) Differentiation between Parkinson disease and other forms of Parkinsonism using support vector machine analysis of susceptibility-weighted imaging (SWI): initial results. Eur Radiol 23:12–19. doi:10.1007/s00330-012-2579-y
Wang Y, Butros SR, Shuai X, Dai Y, Chen C, Liu M, Haacke EM, Hu J, Xu H (2012) Different iron-deposition patterns of multiple system atrophy with predominant parkinsonism and idiopathetic Parkinson diseases demonstrated by phase-corrected susceptibility-weighted imaging. AJNR Am J Neuroradiol 33:266–273. doi:10.3174/ajnr.A2765
Ogisu K, Kudo K, Sasaki M, Sakushima K, Yabe I, Sasaki H, Terae S, Nakanishi M, Shirato H (2013) 3D neuromelanin-sensitive magnetic resonance imaging with semi-automated volume measurement of the substantia nigra pars compacta for diagnosis of Parkinson’s disease. Neuroradiology 55:719–724. doi:10.1007/s00234-013-1171-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This research project was approved by the appropriate Ethics Committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Although patient consent was waived for this retrospective study, MRI and 123I-FP-CIT SPECT studies were performed with informed consent of the patient or the patient’s relatives.
Conflict of interest
We declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kuya, K., Shinohara, Y., Miyoshi, F. et al. Correlation between neuromelanin-sensitive MR imaging and 123I-FP-CIT SPECT in patients with parkinsonism. Neuroradiology 58, 351–356 (2016). https://doi.org/10.1007/s00234-016-1644-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-016-1644-7