Abstract
Introduction
Accurate quantification of hemodynamic parameters using dynamic contrast enhanced (DCE) MRI requires a measurement of tissue T 1 prior to contrast injection (T 1). We evaluate (i) T 1 estimation using the variable flip angle (VFA) and the saturation recovery (SR) techniques and (ii) investigate if accurate estimation of DCE parameters outperform a time-saving approach with a predefined T 1 value when differentiating high- from low-grade gliomas.
Methods
The accuracy and precision of T 1 measurements, acquired by VFA and SR, were investigated by computer simulations and in glioma patients using an equivalence test (p > 0.05 showing significant difference). The permeability measure, K trans, cerebral blood flow (CBF), and - volume, V p, were calculated in 42 glioma patients, using fixed T 1 of 1500 ms or an individual T 1 measurement, using SR. The areas under the receiver operating characteristic curves (AUCs) were used as measures for accuracy to differentiate tumor grade.
Results
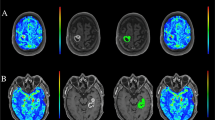
The T 1 values obtained by VFA showed larger variation compared to those obtained using SR both in the digital phantom and the human data (p > 0.05). Although a fixed T 1 introduced a bias into the DCE calculation, this had only minor impact on the accuracy differentiating high-grade from low-grade gliomas, (AUCfix = 0.906 and AUCind = 0.884 for K trans; AUCfix = 0.863 and AUCind = 0.856 for V p; p for AUC comparison > 0.05).
Conclusion
T 1 measurements by VFA were less precise, and the SR method is preferable, when accurate parameter estimation is required. Semiquantitative DCE values, based on predefined T 1 values, were sufficient to perform tumor grading in our study.






Similar content being viewed by others
References
Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT (2007) Angiogenesis in brain tumours. Nat Rev Neurosci 8:610–622
Horsman MR, Siemann DW (2006) Pathophysiologic effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Res 66:11520–11539
Dale BM, Jesberger JA, Lewin JS, Hillenbrand CM, Duerk JL (2003) Determining and optimizing the precision of quantitative measurements of perfusion from dynamic contrast enhanced MRI. J Magn Reson Imaging 18:575–584
Heye T, Davenport MS, Horvath JJ, Feuerlein S, Breault SR, Bashir MR, Merkle EM, Boll DT (2013) Reproducibility of dynamic contrast-enhanced MR imaging. Part I. Perfusion characteristics in the female pelvis by using multiple computer-aided diagnosis perfusion analysis solutions. Radiology 266:801–811
Huang W, Li X, Chen Y, Li X, Chang MC, Oborski MJ, Malyarenko DI, Muzi M, Jajamovich GH, Fedorov A, Tudorica A, Gupta SN, Laymon CM, Marro KI, Dyvorne HA, Miller JV, Barbodiak DP, Chenevert TL, Yankeelov TE, Mountz JM, Kinahan PE, Kikinis R, Taouli B, Fennessy F, Kalpathy-Cramer J (2014) Variations of dynamic contrast-enhanced magnetic resonance imaging in evaluation of breast cancer therapy response: a multicenter data analysis challenge. Transl Oncol 7:153–166
Walker-Samuel S, Parker CC, Leach MO, Collins DJ (2007) Reproducibility of reference tissue quantification of dynamic contrast-enhanced data: comparison with a fixed vascular input function. Phys Med Biol 52:75–89
Haacke EM, Filleti CL, Gattu R, Ciulla C, Al-Bashir A, Suryanarayanan K, Li M, Latif Z, DelProposto Z, Sehgal V, Li T, Torquato V, Kanaparti R, Jiang J, Neelavalli J (2007) New algorithm for quantifying vascular changes in dynamic contrast-enhanced MRI independent of absolute T1 values. Magn Reson Med 58:463–472
Yuan J, Chow SK, Yeung DK, Ahuja AT, King AD (2012) Quantitative evaluation of dual-flip-angle T1 mapping on DCE-MRI kinetic parameter estimation in head and neck. Quant Imaging Med Surg 2:245–253
Larsson C, Kleppesto M, Grothe I, Vardal J, Bjornerud A (2015) T1 in high-grade glioma and the influence of different measurement strategies on parameter estimations in DCE-MRI. J Magn Reson Imaging. doi:10.1002/jmri.24772
Guo JY, Reddick WE, Rosen MA, Song HK (2009) Dynamic contrast-enhanced magnetic resonance imaging parameters independent of baseline T-10 values. Magn Reson Imaging 27:1208–1215
Li J, Yu YM, Zhang YB, Bao SL, Wu CX, Wang XY, Li J, Zhang XP, Hu JN (2009) A clinically feasible method to estimate pharmacokinetic parameters in breast cancer. Med Phys 36:3786–3794
Kaldoudi E, Williams SCR (1993) Relaxation time measurements in NMR imaging. Part I: longitudinal relaxation time. Concepts Magn Reson 5:217–242
Mikkelsen IK, Peters DA, Tietze A (2012) DCE-PWI 3D T1-measurement as function of time or flip angle. ISMRM, Melbourne, Australien
Studler U, White LM, Andreisek G, Luu S, Cheng HL, Sussman MS (2010) Impact of motion on T1 mapping acquired with inversion recovery fast spin echo and rapid spoiled gradient recalled-echo pulse sequences for delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in volunteers. J Magn Reson Imaging 32:394–398
Larsson HB, Courivaud F, Rostrup E, Hansen AE (2009) Measurement of brain perfusion, blood volume, and blood–brain barrier permeability, using dynamic contrast-enhanced T(1)-weighted MRI at 3 tesla. Magn Reson Med 62:1270–1281
Sourbron S, Ingrisch M, Siefert A, Reiser M, Herrmann K (2009) Quantification of cerebral blood flow, cerebral blood volume, and blood–brain-barrier leakage with DCE-MRI. Magn Reson Med 62:205–217
Wansapura JP, Holland SK, Dunn RS, Ball WS Jr (1999) NMR relaxation times in the human brain at 3.0 tesla. J Magn Reson Imaging 9:531–538
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Draganski B, Ashburner J, Hutton C, Kherif F, Frackowiak RS, Helms G, Weiskopf N (2011) Regional specificity of MRI contrast parameter changes in normal ageing revealed by voxel-based quantification (VBQ). Neuroimage 55:1423–1434
Treier R, Steingoetter A, Fried M, Schwizer W, Boesiger P (2007) Optimized and combined T1 and B1 mapping technique for fast and accurate T1 quantification in contrast-enhanced abdominal MRI. Magn Reson Med 57:568–576
Di Giovanni P, Azlan CA, Ahearn TS, Semple SI, Gilbert FJ, Redpath TW (2010) The accuracy of pharmacokinetic parameter measurement in DCE-MRI of the breast at 3 T. Phys Med Biol 55(1):121–132. doi:10.1088/0031-9155/55/1/008
Sung K, Daniel BL, Hargreaves BA (2013) Transmit B1+ field inhomogeneity and T1 estimation errors in breast DCE-MRI at 3 tesla. J Magn Reson Imaging 38:454–459
Wang J, Qiu M, Kim H, Constable RT (2006) T1 measurements incorporating flip angle calibration and correction in vivo. J Magn Reson 182:283–292
Yun TJ, Park CK, Kim TM, Lee SH, Kim JH, Sohn CH, Park SH, Kim IH, Choi SH (2014) Glioblastoma treated with concurrent radiation therapy and temozolomide chemotherapy: differentiation of true progression from pseudoprogression with quantitative dynamic contrast-enhanced MR imaging. Radiology. doi:10.1148/radiol.14132632
Ashton E (2010) Quantitative MR in multi-center clinical trials. J Magn Reson Imaging 31:279–288
Sorensen AG, Batchelor TT, Zhang WT, Chen PJ, Yeo P, Wang M, Jennings D, Wen PY, Lahdenranta J, Ancukiewicz M, di Tomaso E, Duda DG, Jain RK (2009) A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res 69:5296–5300
Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11:83–95
Schabel MC, Parker DL (2008) Uncertainty and bias in contrast concentration measurements using spoiled gradient echo pulse sequences. Phys Med Biol 53:2345–2373
Mouridsen K, Friston K, Hjort N, Gyldensted L, Østergaard L, Kiebel S (2006) Bayesian estimation of cerebral perfusion using a physiological model of microvasculature. Neuroimage 33:570–579
Acknowledgments
This work was supported by the Danish Ministry of Science, Technology and Innovation’s University Investment Grant (MINDLab).
Ethical Standards and Patient Consent
We declare that all human studies have been approved by the Danish National Committee on Health Research Ethics (Journal Number M-20100027) and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 67 kb)
Rights and permissions
About this article
Cite this article
Tietze, A., Mouridsen, K. & Mikkelsen, I.K. The impact of reliable prebolus T 1 measurements or a fixed T 1 value in the assessment of glioma patients with dynamic contrast enhancing MRI. Neuroradiology 57, 561–572 (2015). https://doi.org/10.1007/s00234-015-1502-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-015-1502-z