Abstract
Introduction
The effects of anesthesia are infrequently considered when interpreting pediatric perfusion magnetic resonance imaging (MRI). The objectives of this study were to test for measurable differences in MR measures of cerebral blood flow (CBF) and cerebral blood volume (CBV) between non-sedated and propofol-sedated children, and to identify influential factors.
Methods
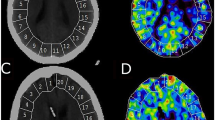
Supratentorial cortical CBF and CBV measured by dynamic susceptibility contrast perfusion MRI in 37 children (1.8–18 years) treated for infratentorial brain tumors receiving propofol (IV, n = 19) or no sedation (NS, n = 18) were compared between groups and correlated with age, hematocrit (Hct), end-tidal CO2 (ETCO2), dose, weight, and history of radiation therapy (RT). The model most predictive of CBF and CBV was identified by multiple linear regression.
Results
Anterior cerebral artery (ACA) and middle cerebral artery (MCA) territory CBF were significantly lower, and MCA territory CBV greater (p = 0.03), in IV than NS patients (p = 0.01, 0.04). The usual trend of decreasing CBF with age was reversed with propofol in ACA and MCA territories (r = 0.53, r = 0.47; p < 0.05). ACA and MCA CBF (r = 0.59, 0.49; p < 0.05) and CBV in ACA, MCA, and posterior cerebral artery territories (r = 0.73, 0.80, 0.52; p < 0.05) increased with weight in propofol-sedated children, with no significant additional influence from age, ETCO2, hematocrit, or RT.
Conclusion
In propofol-sedated children, usual age-related decreases in CBF were reversed, and increases in CBF and CBV were weight-dependent, not previously described. Weight-dependent increases in propofol clearance may diminish suppression of CBF and CBV. Prospective study is required to establish anesthetic-specific models of CBF and CBV in children.




Similar content being viewed by others
References
Hakyemez B et al (2005) High-grade and low-grade gliomas: differentiation by using perfusion MR imaging. Clin Radiol 60(4):493–502
Tsien C et al (2010) Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma. J Clin Oncol 28(13):2293–2299
Lobel U et al (2011) Quantitative diffusion-weighted and dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging analysis of T2 hypointense lesion components in pediatric diffuse intrinsic pontine glioma. AJNR Am J Neuroradiol 32(2):315–322
Heiland S, Wick W, Bendszus M (2010) Perfusion magnetic resonance imaging for parametric response maps in tumors: is it really that easy? J Clin Oncol 28(29):591–592
Salluzzi M, Frayne R, Smith MR (2006) Is correction necessary when clinically determining quantitative cerebral perfusion parameters from multi-slice dynamic susceptibility contrast MR studies? Phys Med Biol 51(2):407–424
Calamante F (2010) Perfusion MRI using dynamic-susceptibility contrast MRI: quantification issues in patient studies. Top Magn Reson Imaging 21(2):75–85
Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Young WL (eds) (2010) Miller’s Anesthesia, vol I, 7th edn. Churchill Livingstone Elsevier, Inc, Philadelphia. ISBN 978-0-443-06959-8
Szabo EZ, Luginbuehl I, Bissonnette B (2009) Impact of anesthetic agents on cerebrovascular physiology in children. Paediatr Anaesth 19(2):108–118
Barthel H et al (1997) Age-specific cerebral perfusion in 4- to 15-year-old children: a high-resolution brain SPET study using 99mTc-ECD. Eur J Nucl Med 24(10):1245–1252
Biagi L et al (2007) Age dependence of cerebral perfusion assessed by magnetic resonance continuous arterial spin labeling. J Magn Reson Imaging 25(4):696–702
Ogawa A et al (1989) Regional cerebral blood flow with age: changes in rCBF in childhood. Neurol Res 11(3):173–176
Chiron C et al (1992) Changes in regional cerebral blood flow during brain maturation in children and adolescents. J Nucl Med 33(5):696–703
Ogawa Y et al (2010) The different effects of midazolam and propofol sedation on dynamic cerebral autoregulation. Anesth Analg 111(5):1279–1284
Schlunzen L et al (2012) Regional cerebral blood flow and glucose metabolism during propofol anaesthesia in healthy subjects studied with positron emission tomography. Acta Anaesthesiol Scand 56(2):248–255
Rigby-Jones AE, Sneyd JR (2011) Propofol and children–what we know and what we do not know. Paediatr Anaesth 21(3):247–254
Shangguan WN et al (2006) Pharmacokinetics of a single bolus of propofol in chinese children of different ages. Anesthesiology 104(1):27–32
Wang C et al (2012) A bodyweight-dependent allometric exponent for scaling clearance across the human life-span. Pharm Res 29(6):1570–1581
Matta BF et al (1999) Direct cerebral vasodilatory effects of sevoflurane and isoflurane. Anesthesiology 91(3):677–680
Cenic A et al (2002) Cerebral blood volume and blood flow responses to hyperventilation in brain tumors during isoflurane or propofol anesthesia. Anesth Analg 94(3):661–6
Sury MR, Smith JH (2008) Deep sedation and minimal anesthesia. Paediatr Anaesth 18(1):18–24
Jain JJ, Glass JO, Reddick WE (2007) Automated arterial input function identification using self organized maps, in SPIE International Symposium on Medical Imaging. Imaging Processing Conference, San Diego, CA, pp 2–17
Sourbron S et al (2004) Choice of the regularization parameter for perfusion quantification with MRI. Phys Med Biol 49(14):3307–3324
Hansen CP (1994) Regularization tools: a matlab package for analysis and solution of discrete ill-posed problems. Numer Algorithms 6(1):1–35
Calamante F et al (1999) Measuring cerebral blood flow using magnetic resonance imaging techniques. J Cereb Blood Flow Metab 19(7):701–735
Calamante F, Gadian DG, Connelly A (2002) Quantification of perfusion using bolus tracking magnetic resonance imaging in stroke: assumptions, limitations, and potential implications for clinical use. Stroke 33(4):1146–1151
Reddick WE et al (1997) Automated segmentation and classification of multispectral magnetic resonance images of brain using artificial neural networks. IEEE Trans Med Imaging 16(6):911–918
Glass JO et al (2004) Improving the segmentation of therapy-induced leukoencephalopathy in children with acute lymphoblastic leukemia using a priori information and a gradient magnitude threshold. Magn Reson Med 52(6):1336–1341
Blumenfeld H (2011) Neuroanatomy Through Clinical Cases. 2nd ed: Sinauer Associates, Inc
Kaisti KK et al (2003) Effects of sevoflurane, propofol, and adjunct nitrous oxide on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology 99(3):603–613
Lee MC et al (2005) Dynamic susceptibility contrast perfusion imaging of radiation effects in normal-appearing brain tissue: changes in the first-pass and recirculation phases. J Magn Reson Imaging 21(6):683–693
Fuss M et al (2000) Radiation-induced regional cerebral blood volume (rCBV) changes in normal brain and low-grade astrocytomas: quantification and time and dose-dependent occurrence. Int J Radiat Oncol Biol Phys 48(1):53–58
Leenders KL et al (1990) Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 113(Pt 1):27–47
Rempp KA et al (1994) Quantification of regional cerebral blood flow and volume with dynamic susceptibility contrast-enhanced MR imaging. Radiology 193(3):637–641
Sakai F et al (1985) Regional cerebral blood volume and hematocrit measured in normal human volunteers by single-photon emission computed tomography. J Cereb Blood Flow Metab 5(2):207–213
Ziegelitz D et al (2009) Absolute quantification of cerebral blood flow in neurologically normal volunteers: dynamic-susceptibility contrast MRI-perfusion compared with computed tomography (CT)-perfusion. Magn Reson Med 62(1):56–65
van Osch MJ, van der Grond J, Bakker CJ (2005) Partial volume effects on arterial input functions: shape and amplitude distortions and their correction. J Magn Reson Imaging 22(6):704–709
Helton KJ et al (2009) Arterial spin-labeled perfusion combined with segmentation techniques to evaluate cerebral blood flow in white and gray matter of children with sickle cell anemia. Pediatr Blood Cancer 52(1):85–91
Han JS et al (2011) Measurement of cerebrovascular reactivity in pediatric patients with cerebral vasculopathy using blood oxygen level-dependent MRI. Stroke 42(5):1261–1269
Acknowledgments
Supported in part by Grant no. CA21765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harreld, J.H., Helton, K.J., Kaddoum, R.N. et al. The effects of propofol on cerebral perfusion MRI in children. Neuroradiology 55, 1049–1056 (2013). https://doi.org/10.1007/s00234-013-1187-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-013-1187-0