Abstract
Introduction
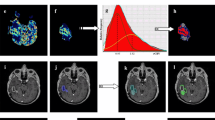
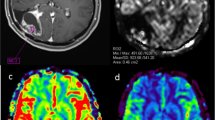
To investigate if perfusion measured with dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) can be used to differentiate radiation necrosis from tumor recurrence in patients with high-grade glioma.
Methods
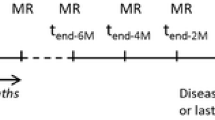
The study was approved by the institutional review board and informed consent was obtained from all subjects. 19 patients were recruited following surgery and radiation therapy for glioma. Patients had contrast enhancing lesions, which during the standard MRI examination could not be exclusively determined as recurrence or radiation necrosis. DCE-MRI was used to measure cerebral blood volume (CBV), blood–brain barrier (BBB) permeability and cerebral blood flow (CBF). Subjects also underwent FDG-PET and lesions were classified as either metabolically active or inactive. Follow-up clinical MRI and lesion histology in case of additional tissue resection was used to determine whether lesions were regressing or progressing.
Results
Fourteen enhancing lesions could be classified as progressing (11) or regressing (three). An empirical threshold of 2.0 ml/100 g for CBV allowed detection of regressing lesions with a sensitivity of 100 % and specificity of 100 %. FDG-PET and DCE-MRI agreed in classification of tumor status in 13 out of the 16 cases where an FDG-PET classification was obtained. In two of the remaining three patients, MRI follow-up and histology was available and both indicated that the DCE-MRI answer was correct.
Conclusion
CBV measurements using DCE-MRI may predict the status of contrast enhancing lesions and give results very similar to FDG-PET with regards to differentiation between tumor recurrence and radiation necrosis.




Similar content being viewed by others
Abbreviations
- AIF:
-
Arterial input function
- BBB:
-
Blood–brain barrier
- CBF:
-
Cerebral blood flow
- CBV:
-
Cerebral blood volume
- DCE-MRI:
-
Dynamic contrast enhanced magnetic resonance imaging
- DSC-MRI:
-
Dynamic susceptibility contrast magnetic resonance imaging
- FDG-PET:
-
18F-fluorodeoxyglucose positron emission tomography
- ICA:
-
Internal carotid artery
- K trans :
-
Transfer constant
- rCBV:
-
Relative CBV
- ROI:
-
Region of interest
- SD:
-
Standard deviation
- TE:
-
Echo time
- WM:
-
White matter
References
Leon SP, Folkerth RD, Black PM (1996) Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer 77:362–372
Folkerth RD (2000) Descriptive analysis and quantification of angiogenesis in human brain tumors. J Neurooncol 50:165–172
Remler MP, Marcussen WH, Tiller-Borsich J (1986) The late effects of radiation on the blood brain barrier. Int J Radiat oncol biol phys 12:1965–1969
Dooms GC, Hecht S, Brant-Zawadzki M et al (1986) Brain radiation lesions. MR-imaging Radiology 158:149–155
Mullins ME, Barest GD, Schaefer PW et al (2005) Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR 26:1967–1672
Cha S, Knopp EA, Johnson G, Wetzel SG, Litt AW, Zagzag D (2002) Intracranial mass lesions: dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology 223:11–29
Nelson SJ (2011) Assessment of therapeutic response and treatment planning for brain tumors using metabolic and physiological MRI. NMR in Biomed 24:734–749
Larsson HB, Hansen AE, Berg HK, Rostrup E, Haraldseth O (2008) Dynamic contrast-enhanced quantitative perfusion measurement of the brain using T1-weighted MRI at 3T. J Magn Reson Imaging 27(4):754–762
Larsson HBW, Courivaud F, Rostrup E, Hansen AE (2009) Measurement of brain perfusion, blood volume, and blood–brain barrier permeability, using dynamic contrast-enhanced T[1]-weighted MRI at 3 Tesla. Magn Reson Med 62:1270–1281
Bjørnerud A, Emblem KE (2010) A fully automated method for quantitative cerebral hemodynamic analysis using DSC-MRI. J cereb flow metab 30:1066–1078
Paulson ES, Schmainda KM (2008) Comparison of dynamic susceptibility-weighted contrast-enhanced MR-methods: Recommendations for measuring relative cerebral blood volume in brain tumors. Radiology 249(2):601–613
Wang SX, Boethius J, Ericson K (2006) FDG-PET on irradiated brain tumor: ten years’ summary. Acta Radiol [1]: 85–90.
Chen W (2007) Clinical applications of PET in brain tumors. J Nucl Med 48:1468–1481
La Fougère C, Suchorska B, Bartenstein P, Kreth F-W, Tonn J-C (2011) Molecular imaging of gliomas with PET: opportunities and limitations. Neuro-oncology 13(8):806–819
Ricci PE, Karis JP, Heiserman JE et al (1998) Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? AJNR 9:407–413
Langleben DD, Segall GM (2000) PET in differentiation of recurrent brain tumor from radiation injury. J Nucl Med 41:1861–1867
Hansen AE, Pedersen H, Rostrup E, Larsson HB (2009) Partial volume effect [PVE] on the arterial input function [AIF] in T1-weighted perfusion imaging and limitations of the multiplicative rescaling approach. Magn Reson Med 62:1055–1059
Lewellen TK, Kohlmyer SG, Miyaoka RS, Kaplan MS (1996) Investigation of the performance of the general electric ADVANCE positron emission tomograph in 3D mode. IEEE Transactions on Nuclear Science 43(4):2199–2206
Patlak CS, Blasberg RG (1985) Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab 5:584–590
Macdonald D, Cascino T, Schold SJ, Cairncross J (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Rostrup E, Knudsen GM, Law I, Holm S, Larsson HBW, Paulson OB (2005) The relationship between cerebral blood flow and volume in humans. Neuroimage 24:1–11
Weybright P, Sundgren PC, Maly P, Hassan DG et al (2005) Differentiation between brain tumor recurrence and radiation injury using MR spectroscopy. Am J Roentgenol 185(6):1471–6
Zeng QC, Li CF, Zang K et al (2007) Multivoxel 3D proton MR spectroscopy in the distinction of recurrent glioma from radiation injury. J Neurooncol 84(1):63–69
Prat R, Galeano I, Lucas A et al (2010) Relative value of magnetic resonance spectroscopy, magnetic resonance perfusion and 2-(18F)fluoro-2-deoxy-d-glucose positron emission tomography for detection of recurrence or grade increase in gliomas. J Clin Neurosci 17(1):50–53
Hu LS, Baxter LC, Smith KA et al (2009) Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. Am J Neuroradiol 30:552–558
Barajas R, Chang JS, Segal MR, Parsa AT, McDermott MW, Berger MS, Cha S (2009) Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 253:486–496
Bobek-Billewicz B, Stasik-Pres G, Majchrzak H, Zarudzki T (2010) Differentiation between brain tumor recurrence and radiation injury using perfusion, diffusion-weighted imaging and MR spectroscopy. Folia Neuropathologica 48:81–92
Sugahara T, Korogi Y, Tomiguchi S et al (2000) Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. Am J neuroradiol 21:901–909
Kim YH, Oh SW, Lim YJ et al (2010) Differentiating radiation necrosis from tumor recurrence in high-grade gliomas: assessing the efficacy of 18F-FDG PET, 11C-methionine PET and perfusion MRI. Clin Neurol Neurosurg 112(9):758–65
Boxerman JL, Schmainda KM, Weisskoff RM (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade whereas uncorrected maps do not. Am J Neuroradiol 27:859–867
Soerensen AG (2008) Perfusion MR imaging: moving forward. Radiology 249(2):416–417
Mills SJ, Soh C, O’connor JP et al (2010) Enhancing fraction in glioma and its relationship to the tumoral vascular microenvironment: a dynamic contrast-enhanced MR imaging study. AJNR Am J Neuroradiol 31(4):726–31
Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ (2010) Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet 9:906–920
Rachinger W, Goetz C, Pöpperl G et al (2005) Positron emission tomography with O-[2-[18F]fluoroethyl]-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery 57(3):505–11
Dandois V, Rommel D, Renard L, Jamart J, Cosnard G (2010) Substitution of 11C-methionine PET by perfusion MRI during the follow-up of treated high-grade gliomas. Preliminary results in clinical practice. J Neuroradiol 37(2):89–97
Forsyth PA, Kelly PJ, Cascino TL, Scheithauer BW, Shaw EG, Dinapoli RP (1995) Radiation necrosis or glioma recurrence: is computer-assisted stereotactic biopsy useful? J Neurosurg 82:436–444
Chorvath M, Boljesikova E, Pruzincova L et al (2009) Post-therapeutical changes in the brain: novel trends in imaging and their influence on external beam radiotherapy. Neoplasma 56(2):156–62
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Larsen, V.A., Simonsen, H.J., Law, I. et al. Evaluation of dynamic contrast-enhanced T1-weighted perfusion MRI in the differentiation of tumor recurrence from radiation necrosis. Neuroradiology 55, 361–369 (2013). https://doi.org/10.1007/s00234-012-1127-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-012-1127-4