Abstract
Introduction
The aim of the present study was to investigate the correlation between local gray matter volume and signal-to-noise ratio on T2-weighted imaging (T2-SNR) of putamen in healthy adults using two tools: voxel-based morphometry (VBM) treating age as a confounding covariate to control for age-related gray matter volume changes and high spatial resolution T1-weighted imaging acquired with a 3.0-T magnetic resonance (3T-MR) scanner.
Methods
Contiguous sagittal T1-weighted images and axial T2-weighted images of the brain were obtained from 1,380 healthy participants. T2-SNR of putamen was defined as A/B, where A is the mean T2-weighted signal intensity (T2-SI) in the right and left sides of putamen, and B is the background noise. The software Statistical Parametric Mapping 5 was used for image segmentation. The association between T2-SNR of putamen and gray matter volume was assessed with VBM, treating age as a confounding covariate.
Results
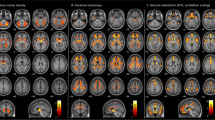
A significant positive correlation was obtained between T2-SNR of putamen and bilateral pre- and postcentral gyrus volume.
Conclusion
To the best of our knowledge, this is the first VBM study to show an age-independent relationship between T2-SNR of putamen and bilateral pre- and postcentral gyrus volumes in healthy adults.


Similar content being viewed by others
References
Ogg RJ, Steen RG (1998) Age-related changes in brain T1 are correlated with iron concentration. Magn Reson Med 40:749–753
Hallgren B, Sourander P (1958) The effect of age on the non-haemin iron in the human brain. J Neurochem 3:41–51
Haacke EM, Cheng NY, House MJ et al (2005) Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 23:1–25
Xu X, Wang Q, Zhang M (2008) Age, gender, and hemispheric differences in iron deposition in the human brain: an in vivo MRI study. Neuroimage 40:35–42
Bartzokis G, Mintz J, Sultzer D et al (1994) In vivo MR evaluation of age-related increases in brain iron. AJNR Am J Neuroradiol 15:1129–1138
Beard JL, Connor JR, Jones BC (1993) Iron in the brain. Nutr Rev 51:157–170
Sachdev P (1993) The neuropsychiatry of brain iron. J Neuropsychiatry Clin Neurosci 5:18–29
Grundke-Iqbal I, Fleming J, Tung YC et al (1990) Ferritin is a component of the neuritic (senile) plaque in Alzheimer dementia. Acta Neuropathol 81:105–110
Connor JR, Menzies SL, St Martin SM et al (1992) A histochemical study of iron, transferrin, and ferritin in Alzheimer's diseased brains. J Neurosci Res 31:75–83
Ehmann WD, Markesbery WR, Alauddin M et al (1986) Brain trace elements in Alzheimer's disease. Neurotoxicology 7:195–206
Parsey RV, Krishnan KR (1998) Quantitative analysis of T2 signal intensities in Alzheimer's disease. Psychiatry Res 82:181–185
Ashburner J, Friston KJ (2000) Voxel-based morphometry—the methods. Neuroimage 11:805–821
Baron JC, Chetelat G, Desgranges B et al (2001) In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer's disease. Neuroimage 14:298–309
Keller SS, Roberts N (2008) Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia 49:741–757
Musen G, Lyoo IK, Sparks CR et al (2006) Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes 55:326–333
Hayano F, Nakamura M, Asami T et al (2009) Smaller amygdala is associated with anxiety in patients with panic disorder. Psychiatry Clin Neurosci 63:266–276
Leube DT, Weis S, Freymann K et al (2008) Neural correlates of verbal episodic memory in patients with MCI and Alzheimer's disease—a VBM study. Int J Geriatr Psychiatry 23:1114–1118
Maguire EA, Gadian DG, Johnsrude IS et al (2000) Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A 97:4398–4403
Hackert VH, den Heijer T, Oudkerk M et al (2002) Hippocampal head size associated with verbal memory performance in nondemented elderly. Neuroimage 17:1365–1372
Folstein MF, Folstein SE, McHugh PR (1975) "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Fazekas F, Chawluk JB, Alavi A et al (1987) MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 149:351–356
Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26:839–851
Aquino D, Bizzi A, Grisoli M et al (2009) Age-related iron deposition in the basal ganglia: quantitative analysis in healthy subjects. Radiology 252:165–172
Sullivan EV, Adalsteinsson E, Rohlfing T et al (2009) Relevance of iron deposition in deep gray matter brain structures to cognitive and motor performance in healthy elderly men and women: exploratory findings. Brain Imaging Behav 3:167–175
Kemp JM, Powell TP (1970) The cortico-striate projection in the monkey. Brain 93:525–546
Kemp JM, Powell TP (1971) The structure of the caudate nucleus of the cat: light and electron microscopy. Philos Trans R Soc Lond B Biol Sci 262:383–401
Jones EG, Coulter JD, Burton H et al (1977) Cells of origin and terminal distribution of corticostriatal fibers arising in the sensory-motor cortex of monkeys. J Comp Neurol 173:53–80
Kwan JY, Jeong SY, Van Gelderen P et al (2012) Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 Tesla MRI and pathology. PLoS One 7:e35241
Schenck JF (2003) Magnetic resonance imaging of brain iron. J Neurol Sci 207:99–102
Duyn JH, van Gelderen P, Li TQ et al (2007) High-field MRI of brain cortical substructure based on signal phase. Proc Natl Acad Sci U S A 104:11796–11801
Gelman N, Gorell JM, Barker PB et al (1999) MR imaging of human brain at 3.0 T: preliminary report on transverse relaxation rates and relation to estimated iron content. Radiology 210:759–767
Bartzokis G, Aravagiri M, Oldendorf WH et al (1993) Field dependent transverse relaxation rate increase may be a specific measure of tissue iron stores. Magn Reson Med 29:459–464
Chen NK, Oshio K, Panych LP et al (2004) Spatially selective T2 and T2* measurement with line-scan echo-planar spectroscopic imaging. J Magn Reson 171:90–96
Zywicke HA, van Gelderen P, Connor JR et al (2002) Microscopic R2* mapping of reduced brain iron in the Belgrade rat. Ann Neurol 52:102–105
Kuhlpeter R, Dahnke H, Matuszewski L et al (2007) R2 and R2* mapping for sensing cell-bound superparamagnetic nanoparticles: in vitro and murine in vivo testing. Radiology 245:449–457
Drayer B, Burger P, Darwin R et al (1986) MRI of brain iron. AJR Am J Roentgenol 147:103–110
Brooks DJ, Luthert P, Gadian D et al (1989) Does signal-attenuation on high-field T2-weighted MRI of the brain reflect regional cerebral iron deposition? Observations on the relationship between regional cerebral water proton T2 values and iron levels. J Neurol Neurosurg Psychiatry 52:108–111
Aoki S, Okada Y, Nishimura K et al (1989) Normal deposition of brain iron in childhood and adolescence: MR imaging at 1.5 T. Radiology 172:381–385
Siemonsen S, Finsterbusch J, Matschke J et al (2008) Age-dependent normal values of T2* and T2′ in brain parenchyma. AJNR Am J Neuroradiol 29:950–955
Schenker C, Meier D, Wichmann W et al (1993) Age distribution and iron dependency of the T2 relaxation time in the globus pallidus and putamen. Neuroradiology 35:119–124
Drayer BP (1989) Basal ganglia: significance of signal hypointensity on T2-weighted MR images. Radiology 173:311–312
Bizzi A, Brooks RA, Brunetti A et al (1990) Role of iron and ferritin in MR imaging of the brain: a study in primates at different field strengths. Radiology 177:59–65
Peran P, Cherubini A, Luccichenti G et al (2009) Volume and iron content in basal ganglia and thalamus. Hum Brain Mapp 30:2667–2675
Rodrigue KM, Haacke EM, Raz N (2011) Differential effects of age and history of hypertension on regional brain volumes and iron. Neuroimage 54:750–759
Imon Y, Yamaguchi S, Katayama S et al (1998) A decrease in cerebral cortex intensity on T2-weighted with ageing images of normal subjects. Neuroradiology 40:76–80
Agartz I, Saaf J, Wahlund LO et al (1991) T1 and T2 relaxation time estimates in the normal human brain. Radiology 181:537–543
Hoult DI, Phil D (2000) Sensitivity and power deposition in a high-field imaging experiment. J Magn Reson Imaging 12:46–67
Allkemper T, Tombach B, Schwindt W et al (2004) Acute and subacute intracerebral hemorrhages: comparison of MR imaging at 1.5 and 3.0 T—initial experience. Radiology 232:874–881
Yao B, Li TQ, Gelderen P et al (2009) Susceptibility contrast in high field MRI of human brain as a function of tissue iron content. Neuroimage 44:1259–1266
Duyn JH (2010) Study of brain anatomy with high-field MRI: recent progress. Magn Reson Imaging 28:1210–1215
Fukunaga M, Li TQ, van Gelderen P et al (2010) Layer-specific variation of iron content in cerebral cortex as a source of MRI contrast. Proc Natl Acad Sci U S A 107:3834–3839
Shmueli K, de Zwart JA, van Gelderen P et al (2009) Magnetic susceptibility mapping of brain tissue in vivo using MRI phase data. Magn Reson Med 62:1510–1522
Takao H, Hayashi N, Ohtomo K (2011) Effect of scanner in longitudinal studies of brain volume changes. J Magn Reson Imaging 34:438–444
Shuter B, Yeh IB, Graham S et al (2008) Reproducibility of brain tissue volumes in longitudinal studies: effects of changes in signal-to-noise ratio and scanner software. Neuroimage 41:371–379
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (Comprehensive Brain Science Network) from the Ministry of Education, Science, Sports and Culture of Japan.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goto, M., Abe, O., Aoki, S. et al. Bilateral pre- and postcentral gyrus volume positively correlates with T2-SNR of putamen in healthy adults. Neuroradiology 55, 245–250 (2013). https://doi.org/10.1007/s00234-012-1126-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-012-1126-5