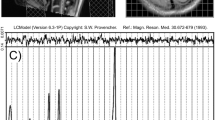
Abstract
Introduction
There are hints for changes in phospholipid membrane metabolism and structure in the brain of adolescents with anorexia nervosa (AN) using either proton (1H) or phosphorus (31P) magnetic resonance spectroscopic imaging (MRSI). We aimed to specify these pathological metabolite changes by combining both methods with additional focus on the neuronal metabolites glutamate (Glu) and N-acetyl-l-aspartate (NAA).
Methods
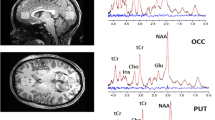
Twenty-one female patients (mean 14.4 ± 1.9 years) and 29 female controls (mean 16 ± 1.6 years) underwent 1H and 31P MRSI at 3 T applied to the centrum semiovale including the anterior cingulate cortex. We assessed gray matter (GM) and white matter (WM) metabolite concentration changes of the frontal and parietal brain measuring choline(Cho)- and ethanolamine(Eth)-containing compounds, Glutamate (Glu) and glutamine (Gln) and their sum (Glx), myoinositol, NAA, and high-energy phosphates.
Results
For 1H MRSI, a clear discrimination between GM and WM concentrations was possible, showing an increase of Glx (p < 0.001), NAA (frontal p < 0.05), pooled creatine (tCr) (p < 0.001), and choline (tCho) (p < 0.05) in the GM of AN patients. The lipid catabolites glycerophosphocholine (p < 0.07) and glycerophosphoethanolamine (p < 0.03) were increased in the parietal region.
Conclusions
Significant changes in GM metabolite concentrations were observed in AN possibly triggered by elevated excitotoxin Glu. Increased tCho may indicate modifications of membrane phospholipids due to increased catabolism in the parietal region. Since no significant changes in phosphorylated choline compounds were found for the frontal region, the tCho increase in this region may hint to fluidity changes.




Similar content being viewed by others
References
Hudson JI, Hiripi E, Pope HG Jr, Kessler RC (2007) The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry 61:348–358
Wentz E, Gillberg IC, Anckarsater H, Gillberg C, Rastam M (2009) Reproduction and offspring status 18 years after teenage-onset anorexia nervosa—a controlled community-based study. Int J Eat Disord 42:483–491
Nielsen S (2001) Epidemiology and mortality of eating disorders. Psychiatr Clin North Am 24:201–214
Brandenburg BM, Andersen AE (2007) Unintentional onset of anorexia nervosa. Eat Weight Disord 12:97–100
Cooper M, Turner H (2000) Underlying assumptions and core beliefs in anorexia nervosa and dieting. Br J Clin Psychol 39:215–218
Fairburn CG, Cooper Z, Doll HA, Welch SL (1999) Risk factors for anorexia nervosa: three integrated case–control comparisons. Arch Gen Psychiatry 56:468–476
Castro J, Lazaro L, Pons F, Halperin I, Toro J (2001) Adolescent anorexia nervosa: the catch-up effect in bone mineral density after recovery. J Am Acad Child Adolesc Psychiatry 40:1215–1221
Mont L, Castro J, Herreros B et al (2003) Reversibility of cardiac abnormalities in adolescents with anorexia nervosa after weight recovery. J Am Acad Child Adolesc Psychiatry 42:808–813
Castro-Fornieles J, Caldu X, Andres-Perpina S, Lazaro L, Bargallo N, Falcon C et al (2010) A cross-sectional and follow-up functional MRI study with a working memory task in adolescent anorexia nervosa. Neuropsychologia 48:4111–4116
Kerem NC, Katzman DK (2003) Brain structure and function in adolescents with anorexia nervosa. Adolesc Med 14:109–118
Fairburn CG, Harrison PJ (2003) Eating disorders. Lancet 361:407–416
Herpertz-Dahlmann BJ, Seitz J, Konrad K (2011) Aetiology of anorexia nervosa: from a “psychosomatic family model” to a neuropsychiatric disorder? Eur Arch Psychiatry Clin Neurosci 261:177–181
Steinhausen HC (2002) The outcome of anorexia nervosa in the 20th century. Am J Psychiatry 159:1284–1293
Suchan B, Busch M, Schulte D, Gronemeyer D, Herpertz S, Vocks S (2010) Reduction of gray matter density in the extrastriate body area in women with anorexia nervosa. Behav Brain Res 206:63–67
Delvenne V, Goldman S, De Maertelaer V, Lotstra F (1999) Brain glucose metabolism in eating disorders assessed by positron emission tomography. Int J Eat Disord 25:29–37
Kojima S, Nagai N, Nakabeppu Y et al (2005) Comparison of regional cerebral blood flow in patients with anorexia nervosa before and after weight gain. Psychiatry Res 140:251–258
Santel S, Baving L, Krauel K, Munte TF, Rotte M (2006) Hunger and satiety in anorexia nervosa: fMRI during cognitive processing of food pictures. Brain Res 1114:138–148
Schonheit B, Meyer U, Kuchinke J, Schulz E, Neumarker KJ (1996) Morphometrical investigations on lamina-V-pyramidal-neurons in the frontal cortex of a case with anorexia nervosa. J Hirnforsch 37:269–280
Uher R, Murphy T, Brammer MJ et al (2004) Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am J Psychiatry 161:1238–1246
Wagner A, Ruf M, Braus DF, Schmidt MH (2003) Neuronal activity changes and body image distortion in anorexia nervosa. Neuroreport 14:2193–2197
Katzman DK, Lambe EK, Mikulis DJ, Ridgley JN, Goldbloom DS, Zipursky RB (1996) Cerebral gray matter and white matter volume deficits in adolescent girls with anorexia nervosa. J Pediatr 129:794–803
Lafon R, Billet M, Billet B (1950) Essential anorexia of young girls and atrophic encephalopathy. Ann Med Psychol (Paris) 108:248–250
Swayze VW, Andersen A, Arndt S, Rajarethinam R, Fleming F, Sato Y, Andreasen NC (1996) Reversibility of brain tissue loss in anorexia nervosa assessed with a computerized Talairach 3-D proportional grid. Psychol Med 26:381–390
Husain MM, Black KJ, Doraiswamy PM et al (1992) Subcortical brain anatomy in anorexia and bulimia. Biol Psychiatry 31:735–738
Gaudio S, Nocchi F, Franchin T, Genovese E, Cannata V, Longo D, Fariello G (2011) Gray matter decrease distribution in the early stages of anorexia nervosa restrictive type in adolescents. Psychiatry Res 191:24–30
Joos A, Kloppel S, Hartmann A et al (2010) Voxel-based morphometry in eating disorders: correlation of psychopathology with grey matter volume. Psychiatry Res 182:146–151
Joos A, Hartmann A, Glauche V et al (2011) Grey matter deficit in long-term recovered anorexia nervosa patients. Eur Eat Disord Rev 19:59–63
Castro-Fornieles J, Bargallo N, Lazaro L, Andres S, Falcon C, Plana MT, Junque C (2009) A cross-sectional and follow-up voxel-based morphometric MRI study in adolescent anorexia nervosa. J Psychiatr Res 43:331–340
Friederich HC, Walther S, Bendszus M et al (2012) Grey matter abnormalities within cortico-limbic-striatal circuits in acute and weight-restored anorexia nervosa patients. Neuroimage 59:1106–1113
McCormick LM, Keel PK, Brumm MC, Bowers W, Swayze V, Andersen A, Andreasen N (2008) Implications of starvation-induced change in right dorsal anterior cingulate volume in anorexia nervosa. Int J Eat Disord 41:602–610
Muhlau M, Gaser C, Ilg R et al (2007) Gray matter decrease of the anterior cingulate cortex in anorexia nervosa. Am J Psychiatry 164:1850–1857
Boghi A, Sterpone S, Sales S, D'Agata F, Bradac GB, Zullo G, Munno D (2011) In vivo evidence of global and focal brain alterations in anorexia nervosa. Psychiatry Res 192:154–159
Lambe EK, Katzman DK, Mikulis DJ, Kennedy SH, Zipursky RB (1997) Cerebral gray matter volume deficits after weight recovery from anorexia nervosa. Arch Gen Psychiatry 54:537–542
Wagner A, Greer P, Bailer UF et al (2006) Normal brain tissue volumes after long-term recovery in anorexia and bulimia nervosa. Biol Psychiatry 59:291–293
Bush G, Luu P, Posner MI (2000) Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4:215–222
Steinglass JE, Walsh BT, Stern Y (2006) Set shifting deficit in anorexia nervosa. J Int Neuropsychol Soc 12:431–435
Frank GK, Bailer UF, Henry S, Wagner A, Kaye WH (2004) Neuroimaging studies in eating disorders. CNS Spectr 9:539–548
Uher R, Brammer MJ, Murphy T, Campbell IC, Ng VW, Williams SC, Treasure J (2003) Recovery and chronicity in anorexia nervosa: brain activity associated with differential outcomes. Biol Psychiatry 54:934–942
Naruo T, Nakabeppu Y, Deguchi D, Nagai N, Tsutsui J, Nakajo M, Nozoe S (2001) Decreases in blood perfusion of the anterior cingulate gyri in anorexia nervosa restricters assessed by SPECT image analysis. BMC Psychiatry 1:2
Takano A, Shiga T, Kitagawa N, Koyama T, Katoh C, Tsukamoto E, Tamaki N (2001) Abnormal neuronal network in anorexia nervosa studied with I-123-IMP SPECT. Psychiatry Res 107:45–50
Jensen JE, Drost DJ, Menon RS, Williamson PC (2002) In vivo brain (31)P-MRS: measuring the phospholipid resonances at 4 Tesla from small voxels. NMR Biomed 15:338–347
Potwarka JJ, Drost DJ, Williamson PC (1999) Quantifying 1H decoupled in vivo 31P brain spectra. NMR Biomed 12:8–14
Mockel R, Schlemmer HP, Guckel F et al (1999) 1H-MR spectroscopy in anorexia nervosa: reversible cerebral metabolic changes. Rofo 170:371–377
Schlemmer HP, Mockel R, Marcus A et al (1998) Proton magnetic resonance spectroscopy in acute, juvenile anorexia nervosa. Psychiatry Res 82:171–179
Hentschel J, Mockel R, Schlemmer HP et al (1999) 1H-MR spectroscopy in anorexia nervosa: the characteristic differences between patients and healthy subjects. Rofo 170:284–289
Roser W, Bubl R, Buergin D, Seelig J, Radue EW, Rost B (1999) Metabolic changes in the brain of patients with anorexia and bulimia nervosa as detected by proton magnetic resonance spectroscopy. Int J Eat Disord 26:119–136
Joos A, Perlov E, Buchert M et al (2011) Magnetic resonance spectroscopy of the anterior cingulate cortex in eating disorders. Psychiatry Res 191:196–200
Grzelak P, Gajewicz W, Wyszogrodzka-Kucharska A, Rotkiewicz A, Stefanczyk L, Goraj B, Rabe-Jablonska J (2005) Brain metabolism alterations in patients with anorexia nervosa observed in 1H-MRS. Psychiatr Pol 39:761–771
Castro-Fornieles J, Bargallo N, Lazaro L, Andres S, Falcon C, Plana MT, Junque C (2007) Adolescent anorexia nervosa: cross-sectional and follow-up frontal gray matter disturbances detected with proton magnetic resonance spectroscopy. J Psychiatr Res 41:952–958
Ohrmann P, Kersting A, Suslow T et al (2004) Proton magnetic resonance spectroscopy in anorexia nervosa: correlations with cognition. Neuroreport 15:549–553
Rzanny R, Freesmeyer D, Reichenbach JR et al (2003) 31P-MR spectroscopy of the brain in patients with anorexia nervosa: characteristic differences in the spectra between patients and healthy control subjects. Rofo 175:75–82
Kato T, Shioiri T, Murashita J, Inubushi T (1997) Phosphorus-31 magnetic resonance spectroscopic observations in 4 cases with anorexia nervosa. Prog Neuropsychopharmacol Biol Psychiatry 21:719–724
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric, Washington, DC
Garner D, Olmsted MP (1984) The Eating Disorder Inventory manual. Psychological Assessment Resources, Odessa
Hattingen E, Magerkurth J, Pilatus U, Hubers A, Wahl M, Ziemann U (2011) Combined (1)H and (31)P spectroscopy provides new insights into the pathobiochemistry of brain damage in multiple sclerosis. NMR Biomed 24:536–546
Gasparovic C, Song T, Devier D et al (2006) Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med 55:1219–1226
Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679
Vanhamme L, van den Boogaart A, Van Huffel S (1997) Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129:35–43
Hattingen E, Magerkurth J, Pilatus U et al (2009) Phosphorus and proton magnetic resonance spectroscopy demonstrates mitochondrial dysfunction in early and advanced Parkinson’s disease. Brain 132:3285–3297
Kreis R (2004) Issues of spectral quality in clinical 1H-magnetic resonance spectroscopy and a gallery of artifacts. NMR Biomed 17:361–381
Buchli R, Duc CO, Martin E, Boesiger P (1994) Assessment of absolute metabolite concentrations in human tissue by 31P MRS in vivo. Part I: cerebrum, cerebellum, cerebral gray and white matter. Magn Reson Med 32:447–452
Hattingen E, Raab P, Franz K, Zanella FE, Lanfermann H, Pilatus U (2008) Myo-inositol: a marker of reactive astrogliosis in glial tumors? NMR Biomed 21:233–241
Pettegrew JW, Kopp SJ, Minshew NJ, Glonek T, Feliksik JM, Tow JP, Cohen M (1987) 31P nuclear magnetic resonance studies of phosphoglyceride metabolism in developing and degenerating brain: preliminary observations. J Neuropathol Exp Neurol 46:419–430
Traber F, Block W, Lamerichs R, Gieseke J, Schild HH (2004) 1H metabolite relaxation times at 3.0 Tesla: Measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. J Magn Reson Imaging 19:537–545
Wockel L, Bertsch T, Koch S et al (2007) The importance of choline and different serum parameters for the course of the anorexia nervosa. Fortschr Neurol Psychiatr 75:402–412
Brenner RE, Munro PM, Williams SC et al (1993) The proton NMR spectrum in acute EAE: the significance of the change in the Cho:Cr ratio. Magn Reson Med 29:737–745
Michaelis T, Merboldt KD, Bruhn H, Hanicke W, Frahm J (1993) Absolute concentrations of metabolites in the adult human brain in vivo: quantification of localized proton MR spectra. Radiology 187:219–227
Miller BL, Chang L, Booth R et al (1996) In vivo 1H MRS choline: correlation with in vitro chemistry/histology. Life Sci 58:1929–1935
Kalyvas A, David S (2004) Cytosolic phospholipase A2 plays a key role in the pathogenesis of multiple sclerosis-like disease. Neuron 41:323–335
Ong WY, Lu XR, Ong BK, Horrocks LA, Farooqui AA, Lim SK (2003) Quinacrine abolishes increases in cytoplasmic phospholipase A2 mRNA levels in the rat hippocampus after kainate-induced neuronal injury. Exp Brain Res 148:521–524
Fonnum F (1984) Glutamate: a neurotransmitter in mammalian brain. J Neurochem 42:1–11
Lee AL, Ogle WO, Sapolsky RM (2002) Stress and depression: possible links to neuron death in the hippocampus. Bipolar Disord 4:117–128
Baker EH, Basso G, Barker PB, Smith MA, Bonekamp D, Horska A (2008) Regional apparent metabolite concentrations in young adult brain measured by (1)H MR spectroscopy at 3 Tesla. J Magn Reson Imaging 27:489–499
Pouwels PJ, Brockmann K, Kruse B, Wilken B, Wick M, Hanefeld F, Frahm J (1999) Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton MRS. Pediatr Res 46:474–485
Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen-Berg H (2010) Age-related changes in grey and white matter structure throughout adulthood. Neuroimage 51:943–951
Hutton C, Draganski B, Ashburner J, Weiskopf N (2009) A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage 48:371–380
Bennett CM, Baird AA (2006) Anatomical changes in the emerging adult brain: a voxel-based morphometry study. Hum Brain Mapp 27:766–777
Blakemore SJ, Choudhury S (2006) Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry 47:296–312
Hudspeth WJ, Pribram KH (1992) Psychophysiological indices of cerebral maturation. Int J Psychophysiol 12:19–29
Killgore WD, Yurgelun-Todd DA (2005) Developmental changes in the functional brain responses of adolescents to images of high and low-calorie foods. Dev Psychobiol 47:377–397
Erecinska M, Zaleska MM, Nissim I, Nelson D, Dagani F, Yudkoff M (1988) Glucose and synaptosomal glutamate metabolism: studies with [15 N] glutamate. J Neurochem 51:892–902
Hattingen E, Lanfermann H, Menon S et al (2009) Combined 1H and 31P MR spectroscopic imaging: impaired energy metabolism in severe carotid stenosis and changes upon treatment. MAGMA 22:43–52
Dringen R, Verleysdonk S, Hamprecht B, Willker W, Leibfritz D, Brand A (1998) Metabolism of glycine in primary astroglial cells: synthesis of creatine, serine, and glutathione. J Neurochem 70:835–840
Hoerst M, Weber-Fahr W, Tunc-Skarka N, Ruf M, Bohus M, Schmahl C, Ende G (2010) Correlation of glutamate levels in the anterior cingulate cortex with self-reported impulsivity in patients with borderline personality disorder and healthy controls. Arch Gen Psychiatry 67:946–954
Moore CM, Frazier JA, Glod CA et al (2007) Glutamine and glutamate levels in children and adolescents with bipolar disorder: a 4.0-T proton magnetic resonance spectroscopy study of the anterior cingulate cortex. J Am Acad Child Adolesc Psychiatry 46:524–534
Stone JM, Day F, Tsagaraki H et al (2009) Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol Psychiatry 66:533–539
Theberge J, Williamson KE, Aoyama N et al (2007) Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry 191:325–334
Buntup D, Skare O, Solbu TT, Chaudhry FA, Storm-Mathisen J, Thangnipon W (2008) Beta-amyloid 25–35 peptide reduces the expression of glutamine transporter SAT1 in cultured cortical neurons. Neurochem Res 33:248–256
Norenberg MD, Bender AS (1995) Astrocyte swelling in liver failure: role of glutamine and benzodiazepines. Acta Neurochir 60:24–27
Videen JS, Michaelis T, Pinto P, Ross BD (1995) Human cerebral osmolytes during chronic hyponatremia. A proton magnetic resonance spectroscopy study. J Clin Invest 95:788–793
Alvin P, Zogheib J, Rey C, Losay J (1993) Severe complications and mortality in mental eating disorders in adolescence. On 99 hospitalized patients. Arch Fr Pediatr 50:755–762
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blasel, S., Pilatus, U., Magerkurth, J. et al. Metabolic gray matter changes of adolescents with anorexia nervosa in combined MR proton and phosphorus spectroscopy. Neuroradiology 54, 753–764 (2012). https://doi.org/10.1007/s00234-011-1001-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-011-1001-9