Abstract
Introduction
The objective of this study was to assess the feasibility and potential clinical applications of diffusion tensor imaging (DTI) and tractography in the normal and pathologic brachial plexus prospectively.
Methods
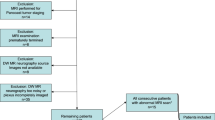
Six asymptomatic volunteers and 12 patients with symptoms related to the brachial plexus underwent DTI on a 1.5T system in addition to the routine anatomic plexus imaging protocol. Maps of the apparent diffusion coefficient (ADC) and of fractional anisotropy (FA), as well as tractography of the brachial plexus were obtained. Images were evaluated by two experienced neuroradiologists in a prospective fashion. Three patients underwent surgery, and nine patients underwent conservative medical treatment.
Results
Reconstructed DTI (17/18) were of good quality (one case could not be reconstructed due to artifacts). In all volunteers and in 11 patients, the roots and the trunks were clearly delineated with tractography. Mean FA and mean ADC values were as follows: 0.30 ± 0.079 and 1.70 ± 0.35 mm2/s in normal fibers, 0.22 ± 0.04 and 1.49 ± 0.49 mm2/s in benign neurogenic tumors, and 0.24 ± 0.08 and 1.51 ± 0.52 mm2/s in malignant tumors, respectively. Although there was no statistically significant difference in FA and ADC values of normal fibers and fibers at the level of pathology, tractography revealed major differences regarding fiber architecture. In benign neurogenic tumors (n = 4), tractography revealed fiber displacement alone (n = 2) or fiber displacement and encasement by the tumor (n = 2), whereas in the malignant tumors, either fiber disruption/destruction with complete disorganization (n = 6) or fiber displacement (n = 1) were seen. In patients with fiber displacement alone, surgery confirmed the tractography findings, and excision was successful without sequelae.
Conclusion
Our preliminary data suggest that DTI with tractography is feasible in a clinical routine setting. DTI may demonstrate normal tracts, tract displacement, deformation, infiltration, disruption, and disorganization of fibers due to tumors located within or along the brachial plexus, therefore, yielding additional information to the current standard anatomic imaging protocols.





Similar content being viewed by others
References
Mori S, Kaufmann WE, Davatzikos C, Stieltjes B, Amodei L, Fredericksen K, Pearlson GD, Melhem ER, Solaiyappan M, Raymond GV, Moser HW, van Zijl PC (2002) Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med 47:215–223
Mori S, Crain BJ, Chacko VP, van Zijl PCM (1999) Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45:265–269
Vargas MI, Delavelle J, Jlassi H, Rilliet B, Viallon M, Becker CD, Lövblad KO (2008) Clinical applications of diffusion tensor tractography of the spinal cord. Neuroradiology 50(1):25–29 Epub 2007 Oct 2
Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G (1996) Diffusion tensor MR imaging of the human brain. Radiology 201(3):637–648
Bammer R, Acar B, Moseley ME (2003) In vivo MR tractography using diffusion imaging. Eur J Radiol 45:223–234
Gillard JH, Papadakis NG, Martin K, Price CJ, Warburton EA, Antoun NM, Huang CL, Carpenter TA, Pickard JD (2001) MR diffusion tensor imaging of white matter tract disruption in stroke at 3 T. Br J Radiol 74(883):642–647
Shimony JS, McKinstry RC, Akbudak E, Aronovitz JA, Snyder AZ, Lori NF, Cull TS, Conturo TE (1999) Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology 212(3):770–784
Wieshmann UC, Clark CA, Symms MR, Franconi F, Barker GJ, Shorvon SD (1999) Reduced anisotropy of water diffusion in structural cerebral abnormalities demonstrated with diffusion tensor imaging. Magn Reson Imaging 17(9):1269–1274
Clark CA, Werring DJ (2002) Diffusion tensor imaging in spinal cord: methods and applications - a review. NMR Biomed 15(7–8):578–586 Review
Wheeler-Kingshott CA, Hickman SJ, Parker GJ, Ciccarelli O, Symms MR, Miller DH, Barker GJ (2002) Investigating cervical spinal cord structure using axial diffusion tensor imaging. Neuroimage 16(1):93–102
Ducreux D, Lepeintre JF, Fillard P, Loureiro C, Tadié M, Lasjaunias P (2006) MR diffusion tensor imaging and fiber tracking in 5 spinal cord astrocytomas. AJNR Am J Neuroradiol 27(1):214–216
Hiltunen J, Suortti T, Arvela S, Seppä M, Joensuu R, Hari R (2005) Diffusion tensor imaging and tractography of distal peripheral nerves at 3 T. Clin Neurophysiol 116(10):2315–2323
Skorpil M, Engström M, Nordell A (2007) Diffusion-direction-dependent imaging: a novel MRI approach for peripheral nerve imaging. Magn Reson Imaging 25(3):406–411
Khalil C, Hancart C, Le Thuc V, Chantelot C, Chechin D, Cotten A (2008) Diffusion tensor imaging and tractography of the median nerve in carpal tunnel syndrome: preliminary results. Eur Radiol 18(10):2283–2291 Epub 2008 Apr 17
Renoux J, Facon D, Fillard P, Huynh I, Lasjaunias P, Ducreux D (2006) MR diffusion tensor imaging and fiber tracking in inflammatory diseases of the spinal cord. AJNR Am J Neuroradiol 27(9):1947–1951
Takahara T, Hendrikse J, Yamashita T, Mali WP, Kwee TC, Imai Y, Luijten PR (2008) Diffusion-weighted MR neurography of the brachial plexus: feasibility study. Radiology 249(2):653–660
Tsuchiya K, Imai M, Tateishi H et al (2007) Neurography of the spinal nerve roots by diffusion tensor scanning applying motion-probing gradients in six directions. Magn Reson Med Sci 6(1):1–5
Viallon M, Vargas MI, Jlassi H, Lövblad KO, Delavelle J (2008) High-resolution and functional magnetic resonance imaging of the brachial plexus using an isotropic 3D T2 STIR (Short Term Inversion Recovery) SPACE sequence and diffusion tensor imaging. Eur Radiol 18(5):1018–1023
Hagmann P, Thiran JP, Jonasson L, Vandergheynst P, Clarke S, Maeder P, Meuli R (2003) DTI mapping of human brain connectivity: statistical fibre tracking and virtual dissection. Neuroimage 19:545–554
Kristoffersen A (2009) Diffusion measurements and diffusion tensor imaging with noisy magnitude data. J Magn Reson Imaging 29(1):237–241
Parker GJ, Haroon HA, Wheeler-Kingshott CA (2003) A framework for a streamline-based probabilistic index of connectivity (PICO) using a structural interpretation of MRI diffusion measurements. J Magn Reson Imaging 18:242–254
Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM (2003) Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 50:1077–1088
Ozturk A, Sasson AD, Farrell JA, Landman BA, da Motta AC, Aralasmak A, Yousem DM (2008) Regional differences in diffusion tensor imaging measurements: assessment of intrarater and interrater variability. AJNR Am J Neuroradiol 29(6):1124–1127
Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S (2007) Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36(3):630–644
Chawla S, Kim S, Wang S, Poptani H (2009) Diffusion-weighted imaging in head and neck cancers. Future Oncol 5(7):959–975
Vandecaveye V, De Keyzer F, Vander Poorten V et al (2009) Head and neck squamous cell carcinoma: value of diffusion-weighted MR imaging for nodal staging. Radiology 251(1):134–146
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vargas, M.I., Viallon, M., Nguyen, D. et al. Diffusion tensor imaging (DTI) and tractography of the brachial plexus: feasibility and initial experience in neoplastic conditions. Neuroradiology 52, 237–245 (2010). https://doi.org/10.1007/s00234-009-0643-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-009-0643-3